Science > Chemistry > Chemical Kinetics > Introduction to Chemical Kinetics
In this article, we shall discuss the concept of chemical kinetics, rate of reactions, and types of reactions on the basis of their rates.
Rate of Reaction:
The branch of chemistry, which deals with the rate of chemical reactions, the factors affecting the rate of reactions and the mechanism of the reaction. is called chemical kinetics.
Importance of Chemical Kinetics:
- It gives an idea that how fast a reaction can occur. using which we know how quickly a medicine is able to work or what is the time required for completion of the reaction.
- In environmental chemistry, it is important to study the ozone balance in the upper atmosphere. The maintenance and depletion of the ozone layer depend on the relative rate of formation and destruction of ozone in the upper atmosphere.
- It is important in catalysis. It is used to solve industrial problems such as the development of catalysts to synthesize new materials.
- It has biological importance. In our body, large protein molecules called enzymes, increase the rate of biological reactions.
- It is important in the food industry. It is used to determine the factors which spoil the food and the rate at which the food is getting spoilt. Hence the probable expiry date or best before the date can be determined.
- The fast setting ceramic material is used for the material for dental fillings.
- It is used to determine the rate at which steel rusts.
- It is used to study the rate at which fuel burns in an automobile engine.
Classification of Chemical Reactions on the Basis of the Rate of the Reaction:
Fast/Instantaneous Reactions (Type – I):
The chemical reaction which completes in less than 1ps (one pieco second) (10-12 s) time, is known as the fast reaction. It is practically impossible to measure the speed of such reactions. The reason for a very fast rate of such reaction is that no chemical bonds are to be broken among the reactants.
e.g., ionic reactions. Organic substitution reactions, neutralization reaction.
Very Slow Reactions (Type – II):
Chemical reactions which complete in a long time from some minutes to some years are called slow reactions. The rates of such reactions are hardly of any physical importance.
e.g. rusting of iron, transformation of carbon into diamond etc.
Moderately Slow Reactions:
Chemical reactions which are intermediate between slow and fast reactions are called moderately slow reactions.
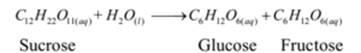
These reactions proceed at a moderate speed which can be measured easily. Mostly these reactions are in molecular nature.
Note:
The rate of chemical reaction can be changed by changing the conditions under which they occur.
Rate of a Reaction:
The rate of a chemical reaction may be defined as the change in concentration of designated species (reactant or product) per unit time. The rate can be measured qualitatively or quantitatively.
Qualitative Rate: The qualitative rate is based on certain visual parameters such as disappearance of reactants, colour change, effervescence etc.
Quantitative Rate: The quantitative rate is based on the rate of decrease in the concentration of any one reactant or the rate of increase in the concentration of any one product.
Average Rate of Reaction:
The average rate of reaction is defined as the change in concentration of reactant or product divided by the time interval over which the change occurs.
Consider a general reaction

Positive sign indicates that the concentration of B is increasing. It is to be noted that the rate of reaction is always positive.
The rate of reaction is expressed in terms of moles per litre per unit time (mol L-1t-1) or molar per unit time (Mt-1). If time is measured in a second then the unit is moles per litre per second (mol L-1s-1) or molar per second (Ms-1). In terms of partial pressure (for gaseous reactions) units is atmosphere per unit time. The average rate of reaction depends on the time interval chosen.
Average Rates of Some Reactions:
Example – 1:

In this case, stoichiometric coefficients of the reactants and products are same, the rate of the reaction is

Example – 2:

In this case, stoichiometric coefficients of H2, N2 and NH3 are 1, 3 and 2 respectively. Hence the rate of the reaction is

Example – 3:

In this case, stoichiometric coefficients of N2O5, NO2 and O2 are 2, 4 and 1 respectively. Hence the rate of the reaction is

General Example:
aA + bB → cC + dD
In this case, stoichiometric coefficients of A, B, C and D are a, b, c and d respectively. Hence the rate of the reaction is

Instantaneous of Rate of a Reaction:
The rate of a reaction at a specific instant is called an instantaneous rate.
If the average rate of reaction is calculated for a shorter and shorter interval of time, a rate at a specific instant can be obtained. The instantaneous rate at the beginning of a reaction is called the initial rate of the reaction.
Consider a general reaction

Example – 1:

In this case, stoichiometric coefficients of H2, N2 and NH3 are 1, 3 and 2 respectively. Hence the rate of the reaction is

General Example:
aA + bB → cC + dD
In this case, stoichiometric coefficients of A, B, C and D are a, b, c and d respectively. Hence the rate of the reaction is

Note:
In chemical kinetics, we deal only with the instantaneous rate of reaction only. Hence the instantaneous rate of reaction is referred as the rate of reaction only.
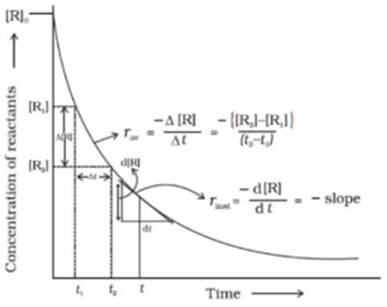
Graphical Representation of Instantaneous and Average Rate of Reaction in terms of Reactants:

Graphical Representation of Instantaneous and Average Rate of Reaction in terms of Products:

Determination of Rate of Reaction:
Determination of Average Rate of Reaction:
A graph is drawn by taking a concentration of species (reactant or product) on y-axis and time on the x-axis.
The average rate of reaction at time t can be obtained by the change in concentration (C2 – C1) of species (reactant or product) in the time interval t1 and t2. (t1 and t2 are equidistant from t) Then the average rate of reaction is calculated using following formula.

Determination of Instantaneous Rate of Reaction:
A graph is drawn by taking the concentration of species (reactant or product) on y-axis and time on the x-axis.
The instantaneous rate of reaction at time t can be obtained by drawing a tangent at time t and finding its slope at that point. The slope of the tangent to the curve at that point gives an instantaneous rate of reaction.
Reaction Life Time:
It is defined as the time taken by a reaction to proceed to 98% of completion. Shorter the life time, faster is the reaction. It is used to compare the rate of reactions.
Rate Laws and Rate Constant:
Law of Mass Action:
This law was given by Goldberg and Waage in 1864. It states that “at a given temperature, the rate of reaction at a particular instant is proportional to the product of the active masses of the reactants at that instant raised to powers which are numerically equal to the numbers of their respective molecules in the stoichiometric equation describing the reaction.”
Explanation:
Consider general reaction
aA + bB → cC + dD
By law of mass action
Rate ∝ [A]a [B]b
∴ Rate = K [A]a [B]b
Rate Law:
The rate of chemical reaction is found to be proportional to the molar concentrations of the reactants raised to simple powers.
Let us consider a general reaction
aA + bB → Products
Let us assume the rate of reaction depends on[A]x and [B]y.
Thus, Rate ∝ [A]x [B]y
Rate = K [A]x [B]y …………… (1)
But instantaneous rate of reaction is given by

This relation is known as the differential rate law or simply rate law. Where k is a constant called specific reaction rate constant or simply rate constant.
Definition:
The rate law is defined as an experimentally determined equation that expresses the rate of a chemical reaction in terms of molar concentrations of the reactants.
Rate Constant:
By rate law we have, Rate = K [A]x [B]y
If [A] = 1 and [B] = 1, Then
Rate = k = Rate Constant
The rate constant is defined as the rate of reaction would have if all the concentrations were set equal to unity.
Characteristics of Rate Constant:
- The values of the rate constant give an idea about the speed of the reaction. Greater the value of the rate constant, faster is the reaction.
- Each reaction has a definite value of the rate constant at a particular temperature.
- The value of the rate constant depends on temperature.
- The value of rate constant is independent of the concentration of reacting species.
- The value of rate constant depends on nature of reactant, the presence of catalyst, solvent and pH of a solution.
- The unit of rate constant depends on the order of the reaction. In general unit of rate constant is

Where n is the order of reaction.
Notes:
- The value of x and y in the rate law are not necessarily equal to the stoichiometric coefficients (a and b) of A and B.
- Values of x and y are determined experimentally.
Example to Illustrate Rate Law:
Example:
In the reaction, NO2(g) + CO(g) → NO(g) + CO2(g)
The rate of reaction is experimentally found to proportional
to [NO2]² and independent of [CO]. Thus x = 2 and y = 0
Thus the rate law of reaction is

Applications of Rate Law:
- The rate law can be used to estimate the rate of a reaction for any given composition of the reaction mixture.
- It can be used to estimate the concentration of reactants and products at any time during the course of reaction.\
- The rate law is useful for prediction of the mechanism of a complex reaction.
Characteristics of Rate of Reaction:
- It is the speed at which the reactants are converted into products at any instant of time.
- It is dependent on the concentrations of the reactant species at that instant.
- As the reaction proceeds the rate of reaction decreases.
Characteristics of Rate Constant:
- It is constant of proportionality in rate law expression.
- It is independent of the concentration of reactants.
- It is constant throughout the reaction i.e. it is independent of the progress of the reaction.