Science > Chemistry > Atomic Structure > Dual Nature of Radiations
Light and other electromagnetic radiations have dual nature viz: the particle nature and the wave nature.
Wave Nature of Radiations:
Radiation is the form of energy, which can be transferred from one point to another point in space. Radiations are considered to be transmitted by wave motion. A wave consist of crests and troughs.
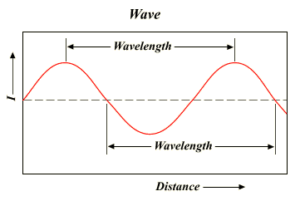
- The distance between two consecutive troughs or consecutive crests is called as the wavelength (λ). its S.I. unit is m. and practical unit is angstrom (Å).
- The number of waves passing through any point in unit time (1 sec) is known as frequency (υ). It is Expressed in cycles per second (cps) or Hz.
- Distance travelled by the wave in one second is called the velocity of the wave. denoted by ‘c’. Expressed in m/sec.
Electromagnetic Nature of Radiations:
Electromagnetic nature of radiations is explained by James Maxwell (1870). He suggested that when electrically charged particles move with an acceleration alternating electrical and magnetic fields are produced and transmitted. These fields are transmitted in the forms of waves called electromagnetic waves or electromagnetic radiation. The oscillating electric and magnetic fields produced by oscillating charged particles are perpendicular to each other and both are perpendicular to the direction of propagation of the wave.
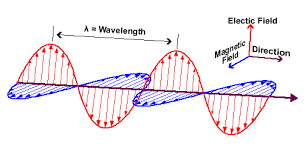
The arrangement of different electromagnetic radiations in order of increasing wavelength is called electromagnetic spectrum. Unlike sound waves or water waves, electromagnetic waves do not require medium and can move in vacuum.
Particle Nature of Light or Planck’s Quantum Theory:
Quantum theory was given by Max Planck in 1900. Its important postulates are
- Absorption and emission of radiant energy does not takes place continuously but it takes place in the form of packets of energy
called quanta. Quantum of light is called a photon.
- Each quanta has definite amount of energy which depends upon frequency of radiation. The relation is E = hυ, Where E is energy of photon, h is Planck’s constant and υ is the frequency of the radiation.
- Quantum is the smallest denomination of energy. The quantum of energy is always an integer.
- Energy less than quantum can never be absorbed or emitted. It can be emitted as whole number multiple of quantum I.e. 1hυ, 2hυ, 3hυ etc
Particle nature could explain the black body radiation and photoelectric effect satisfactorily but on the other hand, it was not consistent with the known wave behaviour of light which could account for the phenomena of interference and diffraction. The only way to resolve the dilemma was to accept the idea that light possesses both particle and wave-like properties, i.e., light has dual behaviour.
Dual Nature of Radiations:
Depending on the experiment, we find that light behaves either as a wave or as a stream of particles. Whenever radiation interacts with the matter, it displays particle like properties in contrast to the wavelike properties (interference and diffraction), which it exhibits when it propagates. Thus light has dual nature. Some microscopic particles like electrons also exhibit this wave-particle duality.
De-Broglie Equation:
de Broglie in 1924 proposed that matter, like radiation, should also exhibit dual behaviour i.e., both particle and wave like properties. This means that just as the photon has momentum as well as wavelength, electrons should also have momentum as well as wavelength.
De Broglie, from this analogy, gave the following relation between the wavelength (λ) and momentum (p) of a material particle.

where m is the mass of the particle, v its velocity and p its momentum.
Notes:
- De Broglie’s prediction was confirmed experimentally when it was found that an electron beam undergoes diffraction, a phenomenon characteristic of waves. This fact has been put to use in making an electron microscope, which is based on the wave-like the behaviour of electrons just as an ordinary microscope utilizes the wave nature of light. An electron microscope is a powerful tool in modern scientific research because it achieves a magnification of about 15 million times.
- It needs to be noted that according to de Broglie, every object in motion has a wave character. The wavelengths associated with ordinary objects are so short (because of their large masses) that their wave properties cannot be detected.
- The wavelengths associated with electrons and other subatomic particles (with very small mass) can, however, be detected experimentally.