Science > Chemistry > Chemical Kinetics > Molecularity of Reaction and Catalysis
In this article, we shall study the molecularity of reaction and catalysis.
The Concept of Elementary Reactions:
Many reactions that follow a simple rate law are actually taking place in series of steps. These reactions are called complex reactions. Each step in a complex reaction is called elementary reaction. Thus complex reaction can be broken down into the elementary reactions.
An elementary reaction is defined as a reaction that takes place in a single step and can’t be broken down further into simplest chemical reactions.
Illustration:
Consider complex reaction
3 ClO–(aq) → ClO3–(aq) + 2Cl–(aq)
Actually, this reaction takes place in two steps. Thus there are two elementary reactions.
Step-1:
2 ClO–(aq) → ClO2–(aq) + Cl–(aq) (Bimolecular reaction)
Step – 2:
ClO2–(aq) + ClO–(aq) → ClO3–(aq) + Cl–(aq) (Bimolecular reaction)
The sum of the two reactions gives the overall reaction.
Molecularity of Elementary Reactions:
The molecularity of an elementary reaction is defined as the number of reaction molecules taking part in the reaction.
Example of Unimolecular Reaction:
O3(g) → O2(g) + O(g)
C2H5I(g) → C2H4(g) + HI(g)
Example of Bimolecular Reaction:
O3(g) + O(g) → 2O2(g)
2NO2(g) → 2NO(g) + O2(g)
Distinguishing Between Molecularity and Order of Reaction:
Molecularity of a Reaction:
- The molecularity of a reaction is defined as the number of reaction molecules taking part in the reaction.
- Molecularity is always a whole number.
- It is a theoretical property indicating the number of molecules involved in each act leading to the reaction.
- It does not change with experimental conditions.
- It is the property of elementary reaction and has no meaning for a complex reaction.
Order of a Reaction:
- The overall order of the reaction is defined as the sum of the exponents to which the concentration terms in the rate law are raised.
- order of reaction may be an integer, fraction, or zero.
- It is a purely experimental property indicating the dependence of the observed reaction rate on the concentration of the reactants.
- It may change with experimental conditions.
- It is the property of both elementary and complex reactions.
Multistep Reactions:
A multistep reaction is a reaction involving two or more steps. Consider the reaction
A → C
It consists of two steps.
First step: A → B
Second step: B → C
In the above reaction, B is intermediate. The Intermediate is the product of the first step and reactant of the second step.
Reaction Intermediates:
The additional species other than the reactants or products formed in the mechanism during the progress of a reaction is called reaction intermediate.
Characteristics of Intermediate:
- They may be stable or unstable.
- The number of Intermediates in a reaction = The number of Steps – 1
- Thus reaction involving two steps will have one intermediate and one step reaction will have no intermediate.
- The intermediates appear in mechanism but do not appear in overall reaction because they are produced in one step and consumed in another step.
- The concentration of reaction intermediates is very small, hence cannot be determined easily.
- The rate of reaction is independent of the concentration of intermediates.
- The life period of reaction intermediates is very small hence they cannot be isolated.
Catalysis:
Catalyst and its Effect on the Rate of Reaction:
A catalyst is a substance, added to the reactants, that increases the rate of reaction without itself being consumed in the reaction
Example: In preparation of O2 from KClO3 in laboratories MnO2 is used as a catalyst.
Characteristics of Catalyst:
- The catalyst does not appear in an overall reaction because they are consumed in one step and regenerated in another step.
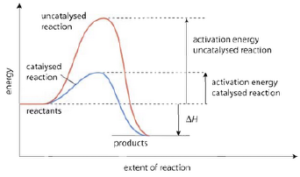
- A catalyst lowers the activation energy of a reaction.
- In presence of a catalyst the height of the energy barrier decreases. Thus the number of molecules the possess the minimum kinetic energy increases.
- Chemically, the catalyst remains unchanged during a reaction.
- Catalyst does not change the quantity of the product.
- A catalyst is specific, which means different chemical reactions may have a different catalyst.
- Just a small amount needed to achieve a big increase in the rate of reaction.
- More amount of catalyst used can further increase the rate of reaction.
- A catalyst in powder form can further increase the rate of reaction.
- A catalyst may undergo a physical change in a reaction.
Distinguishing Between Catalyst and Reaction Intermediate:
Catalyst:
- A catalyst is a substance, added to the reactants, that increases the rate of the reaction without itself being consumed in the reaction.
- A catalyst increases the rate of a reaction.
- A catalyst is present at the start of the reaction.
- A catalyst is consumed in one step and regenerated in the subsequent step.
- The concentration of catalyst may appear in rate law.
- Catalysts are stable under ordinary conditions.
Intermediate:
- The additional species other than the reactants or products formed in the mechanism during the progress of a reaction is called reaction intermediate.
- Intermediate has no effect on the rate of reaction.
- An intermediate exist during the mechanism of the reaction.
- An intermediate is produced in one step and consumed in the subsequent step.
- The concentration of intermediate does not appear in rate law.
- Intermediates are highly unstable and ha a short life.
Note:
Both catalyst and intermediate do not appear in overall reaction.
Rate Determining Step (R.D.S.)
In a multistep reaction, rate of overall reaction depends upon the rate of the slowest step. This slowest step is called rate determining step.
Example : Consider substitution reaction
R-X + Y → R-Y + X
Substrate Reagent Product Living group
This reaction takes place as follows
First Step:
R – X → R+ + : X–
Second Step:
R+ + : Y– → R Y
In above case rate of overall reaction depends on the rate of the first step. Hence the first step is labelled as R.D.S.
The overall reaction cannot take place faster than the rate of rate determining step. Hence RDS step determines rate of overall reaction. As RDS is elementary reaction, the rate law can be determined from its stoichiometric equation. In rate law the exponents are equal to the coefficient of balanced equation for the step.