Science > Chemistry > Atomic Structure > Problems Based on Atomic Number, Mass Number, and Neutron Number
In this article, we shall study to solve problems based on the calculation of atomic number, atomic mass number, and neutron number
Atomic number (Z) :
The number of protons (positive charge) present in the nucleus of an atom of a particular element is called the atomic number of that element. It is denoted by letter ‘Z’.
Neutron number (N):
The number of neutrons present in the nucleus of an atom is known as neutron number. It is denoted by ‘N’
Mass number (A):
The total number of protons and neutrons present in the nucleus of an atom of the element is called mass number. The mass number is denoted as ‘A’.
A = Z + N
Example 01:
Calculate the number of electrons, protons, and neutrons in the following atoms.

Atomic Number = Z = 15
Atomic Mass Number = A = 31
Number of protons = atomic number = 15
Number of electrons = atomic number = 15
Neutron number = Number of neutrons = A – Z = 31 – 15 = 16
Number of nucleons = Atomic mass number = 31
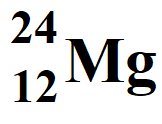
Atomic Number = Z = 12
Atomic Mass Number = A = 24
Number of protons = atomic number = 12
Number of electrons = atomic number = 12
Neutron number = Number of neutrons = A – Z = 24 – 12 = 12
Number of nucleons = Atomic mass number = 24
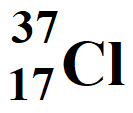
Atomic Number = Z = 17
Atomic Mass Number = A = 37
Number of protons = atomic number = 17
Number of electrons = atomic number = 17
Neutron number = Number of neutrons = A – Z = 37 – 17 = 20
Number of nucleons = Atomic mass number = 37

Atomic Number = Z = 18
Atomic Mass Number = A = 40
Number of protons = atomic number = 18
Number of electrons = atomic number = 18
Neutron number = Number of neutrons = A – Z = 40 – 18 = 22
Number of nucleons = Atomic mass number = 40

Atomic Number = Z = 35
Atomic Mass Number = A = 80
Number of protons = atomic number = 35
Number of electrons = atomic number = 35
Neutron number = Number of neutrons = A – Z = 80 – 35 = 45
Number of nucleons = Atomic mass number = 80

Atomic Number = Z = 20
Atomic Mass Number = A = 40
Number of protons = atomic number = 20
Number of electrons = atomic number = 20
Neutron number = Number of neutrons = A – Z = 40 – 20 = 20
Number of nucleons = Atomic mass number = 40

Atomic Number = Z = 6
Atomic Mass Number = A = 13
Number of protons = atomic number = 6
Number of electrons = atomic number = 6
Neutron number = Number of neutrons = A – Z = 13 – 6 = 7
Number of nucleons = Atomic mass number = 13

Atomic Number = Z = 8
Atomic Mass Number = A = 16
Number of protons = atomic number = 8
Number of electrons = atomic number = 8
Neutron number = Number of neutrons = A – Z = 16 – 8 = 8
Number of nucleons = Atomic mass number = 16

Atomic Number = Z = 26
Atomic Mass Number = A = 56
Number of protons = atomic number = 26
Number of electrons = atomic number = 26
Neutron number = Number of neutrons = A – Z = 56 – 26 = 30
Number of nucleons = Atomic mass number = 56

Atomic Number = Z = 38
Atomic Mass Number = A = 88
Number of protons = atomic number = 38
Number of electrons = atomic number = 38
Neutron number = Number of neutrons = A – Z = 88 – 38 = 50
Number of nucleons = Atomic mass number = 88
Example 02:
Calculate the number of electrons, protons, and neutrons in the following species.

Atomic Number = Z = 15
Atomic Mass Number = A = 31
Number of protons = atomic number = 15
P3- → P + 3 e–
Number of electrons = 15 + 3 = 18
Number of neutrons = A – Z = 31 – 15 = 16
Number of nucleons = Atomic mass number = 31

Atomic Number = Z = 35
Atomic Mass Number = A = 80
Number of protons = atomic number = 35
Br– → Br + e–
Number of electrons = 35 + 1= 36
Number of neutrons = A – Z = 80 – 35 = 45
Number of nucleons = Atomic mass number = 80

Atomic Number = Z = 20
Atomic Mass Number = A = 40
Number of protons = atomic number = 20
Ca2+ → Ca – 2e–
Number of electrons = 20 – 2= 18
Number of neutrons = A – Z = 40 – 20 = 20
Number of nucleons = Atomic mass number = 40
H3PO4
Hydrogen (H):
Atomic Number = Z = 1
Atomic Mass Number = A = 1
Number of protons per atom= atomic number = 1
Number of electrons per atom = Z = 1
Number of neutrons per atom = A – Z = 1 – 1 = 0
There are 3 hydrogen atoms in the molecule
Total number of protons in 3 hydrogens = 1 x 3 = 3
Total number of electrons in 3 hydrogens = 1 x 3 = 3
Total number of neutrons in 3 hydrogens = 0 x 3 = 0
Phosphorous (P):
Atomic Number = Z = 15
Atomic Mass Number = A = 31
Number of protons per atom= atomic number = 15
Number of electrons per atom = Z = 15
Number of neutrons per atom = A – Z = 31 – 15 = 16
There is 1 phosphorous atom in the molecule
Total number of protons in 1 phosphorous = 15 x 1 = 15
Total number of electrons in 1 phosphorous = 15 x 1 = 15
Total number of neutrons in 1 phosphorous = 16 x 1 = 16
Oxygen (O):
Atomic Number = Z = 8
Atomic Mass Number = A = 16
Number of protons per atom= atomic number = 8
Number of electrons per atom = Z = 8
Number of neutrons per atom = A – Z = 16 – 8 = 8
There are 4 oxygen atom in the molecule
Total number of protons in 4 oxygen= 8 x 4 = 32
Total number of electrons in 4 oxygen = 8 x 4 = 32
Total number of neutrons in 4 oxygen = 8 x 4 = 32
Ans:
Total number of protons in H3PO4 = 3 + 15 + 32 = 50
Total number of electrons in H3PO4 = 3 + 15 + 32 = 50
Total number of neutrons in H3PO4 = 0 + 16 + 32 = 48
NH4+
Nitrogen (N):
Atomic Number = Z = 7
Atomic Mass Number = A = 14
Number of protons per atom= atomic number = 14
Number of electrons per atom = Z = 14
Number of neutrons per atom = A – Z = 14 – 7 = 7
Hydrogen (H):
Atomic Number = Z = 1
Atomic Mass Number = A = 1
Number of protons per atom= atomic number = 20
Number of electrons per atom = Z = 1
Number of neutrons per atom = A – Z = 1 – 1 = 0
There are 4 hydrogen atoms in the species
Total number of protons in 3 hydrogens = 1 x 4 = 4
Total number of electrons in 3 hydrogens = 1 x 4 = 4
Total number of neutrons in 3 hydrogens = 0 x 4 = 0
Total number of protons in NH4+ = 7 + 4 = 11
NH4+ → NH4 – e–
Total number of electrons in NH4+ = 7 + 4 – 1 = 10
Total number of neutrons in NH4+ = 7 + 4 = 11
ClO3–
Chlorine (Cl):
Atomic Number = Z = 17
Atomic Mass Number = A = 37
Number of protons per atom= atomic number = 17
Number of electrons per atom = Z = 17
Number of neutrons per atom = A – Z = 37 – 17 = 20
Oxygen (O):
Atomic Number = Z = 8
Atomic Mass Number = A = 16
Number of protons per atom= atomic number = 8
Number of electrons per atom = Z = 8
Number of neutrons per atom = A – Z = 16 – 8 = 8
There are 3 oxygen atom in the species
Total number of protons in 4 oxygen= 8 x 3 = 24
Total number of electrons in 4 oxygen = 8 x 3 = 24
Total number of neutrons in 4 oxygen = 8 x 3 = 24
Total number of protons in ClO3– = 17 + 24 = 41
ClO3– → ClO3 + e–
Total number of electrons in ClO3– = 17 + 24 + 1 = 42
Total number of neutrons in ClO3– = 20 + 24 = 44
Example 03:
Complete the table
| Sr. No. | Symbol | Mass No. | Atomic No. | Protons | Neutrons | Electrons |
| 1 | Zn | 64 | 30 | – | – | – |
| 2 | Sr2+ | 90 | 38 | – | – | – |
| 3 | Te | – | – | 43 | 56 | – |
| 4 | Br– | – | – | – | 44 | 36 |
| 5 | N | – | – | – | 7 | 7 |
| 6 | Ca2+ | – | 20 | 20 | 20 | – |
| 7 | O | 16 | 8 | – | – | – |
Solution:
Zn
Atomic Number = Z = 30
Atomic Mass Number = A = 64
Number of protons = atomic number = 30
Number of electrons = atomic number = 30
Number of neutrons = A – Z = 64 – 30 = 34
Sr2+
Atomic Number = Z = 38
Atomic Mass Number = A = 90
Number of protons = atomic number = 38
Sr2+ → Sr – 2e–
Number of electrons = 38 – 2 = 36
Number of neutrons = A – Z = 90 – 38 = 52
Te
Number of protons = 43
Number of Neutrons = N = 56
Atomic number =Number of protons = Z = 43
Atomic mass number = Z + N = 43 + 56 = 99
Number off electrons = Atomic number = 43
Br–
Number of neutrons = N = 44
Number of electrons = 36
Br– → Br + e–
Number of protons = 36 – 1 = 35
Atomic Number = Number of protons = Z = 35
Atomic mass number = Z + N = 35 + 44 = 79
N
Number of neutrons = N = 7
Number of electrons = 7
Number of protons = 7
Atomic Number = Number of protons = Z = 7
Atomic mass number = Z + N = 7 + 7 = 14
Ca2+
Atomic Number = Z = 20
Number of neutrons = N = 20
Atomic Mass Number = Z + N = 20 + 20 = 40
Number of protons = atomic number = 20
Ca2+ → Ca – 2e–
Number of electrons = 20 – 2 = 18
O
Atomic Number = Z = 8
Atomic Mass Number = A = 16
Number of protons = atomic number = 8
Number of electrons = atomic number = 8
Number of neutrons = A – Z = 16 – 8 = 8
The completed table is as follows:
| Sr. No. | Symbol | Mass No. | Atomic No. | Protons | Neutrons | Electrons |
| 1 | Zn | 64 | 30 | 30 | 34 | 30 |
| 2 | Sr2+ | 90 | 38 | 38 | 52 | 36 |
| 3 | Te | 99 | 43 | 43 | 56 | 43 |
| 4 | Br– | 79 | 35 | 35 | 44 | 36 |
| 5 | N | 14 | 7 | 7 | 7 | 7 |
| 6 | Ca2+ | 40 | 20 | 20 | 20 | 18 |
| 7 | O | 16 | 8 | 8 | 8 | 8 |
Example 04:
The numbers of electrons, protons, and neutrons in a monoatomic species are equal to 36, 35, and 45 respectively. Assign proper symbol.
Solution:
Number of electrons = 36
Number of protons = Atomic number = Z = 35
Number of neutrons = N = 45
Atomic mass number = A = Z + N = 35 + 45 = 80
Charge on species = Z – Number of electrons = 35 – 36 = -1
The species is
![]()
Example 05:
The numbers of electrons, protons, and neutrons in a monoatomic species are equal to 18, 16, and 16 respectively. Assign proper symbol.
Solution:
Number of electrons = 18
Number of protons = Atomic number = Z = 16
Number of neutrons = N = 16
Atomic mass number = A = Z + N = 16 + 16 = 32
Charge on species = Z – Number of electrons = 16 – 18 = -2
The species is

Example 06:
Find the number of electrons in fluorine atom, fluorine molecule, and fluoride ion. The atomic number and mass number of fluorine are 9 and 19 respectively.
Solution:
Atomic number = Z = 9
Atomic mass number A = 19
Fluorine Atom (F):
Number of protons = Atomic number = 9
Number of Protons = Atomic number = 9
Number of electrons = Atomic number = 9
Number of neutrons = a – z = 19 – 9 =10
Fluorine Molecule (F2):
There are 2 atoms in a molecule of fluorine
Number of protons in fluorine molecule= 9 x 2 = 18
Number of electrons in fluorine molecule= 9 x 2 = 18
Number of neutrons in fluorine molecule= 10 x 2 = 20
Fluoride Ion (F–):
Number of Protons = Atomic number = 9
F– → F + e–
Number of electrons = 9 + 1 = 10
Number of neutrons = a – z = 19 – 9 =10
Example 07:
An isotope of atomic mass 24 had 12 neutrons in its nucleus. What is its atomic number? Represent the isotope in symbolic form.
Solution:
Atomic mass number = A = 24
Number of neutrons = N = 12
Number of protons = A – Z = 24 – 12= 12
Atomic number = Number of protons = Z = 12
The element is
![]()
Isotopes, Isotones, and Isobars
- Different atoms of the same element having the same atomic number but having different mass numbers are known as isotopes.
- Atoms of the different elements having a different atomic number but having the same mass numbers are known as isobars.
- Atoms of the different elements having the different atomic number, different mass number but having the same neutron number are known as isotones.
Example 08:
Write the complete symbol for the atom with the given atomic number (Z) and atomic mass (A):
- Z = 17 and A = 35

- Z = 92, A = 233

- Z = 4, A = 9

Example 09:
Give an isobar, an isotone, and an isotope of ![]()
| Isobar |  |
| Isotone |  |
| Isotope |  |