Science > Chemistry > Chemical Thermodynamics and Energetics > Bond Enthalpy
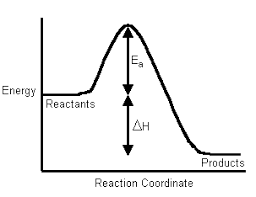
In this article, we shall study the concept of bond enthalpy. During the formation of the bond, energy is evolved or released. Thus the bond formation reaction is exothermic. Due to the release of energy molecule formed is at a low energy level and more stable than isolated atoms. The energy released when a covalent bond is formed is called the energy of bond formation.
Bond Enthalpy:
Chemical reactions involve the breaking of bonds in the reactants and the formation of new bonds in the products with the liberation of energy. Whenever a chemical bond is formed, in a molecule, the energy is liberated and therefore to break the same bond an equivalent amount of energy must be given (supplied) to the molecule. Hence bond enthalpy can be defined as “the average amount of energy per mole required to break a particular bond in a gaseous molecule producing free gaseous atoms or radicals”.
Example:
CH4(g) → C(g) + 4H(g), ΔH° = 1664 KJ mol-1
The meaning of the above equation is that to break 1 mole of methane in the gaseous state into carbon (in a gaseous state) and hydrogen in a gaseous state, 1664 KJ of energy is required. This is the energy required to break 4 C-H bonds in Methane.
Note: It should be noted that in the above case the products are C(g) and 4H(g) and not 2H2(g) (g), C(s).
Now, a molecule of methane contains 4 C — H bonds. Hence the average C —H bond energy in methane = 1664 /4 = 416 KJ mol-1.
Bond Dissociation Enthalpy:
It is defined as the energy required per mole to break a specific bond in a specific molecule, in a gaseous state.
For diatomic molecules, bond energy and bond dissociation energy will be the same. But for polyatomic molecules bond energy and bond dissociation energy will be different.
Example of Poly Atomic Molecule:
The different bond dissociation energies involved in methane are as follows :
CH4(g) → CH3(g) + H(g), DΔH°CH3-C = 435 KJ mol-1
CH3(g) → CH2(g) + H(g), DΔH°CH2-C = 443 KJ mol-1
CH2(g) → CH(g) + H(g), DΔH°CH-C = 443 KJ mol-1
CH(g) → C(g) + H(g), D ΔH°CH-C = 343 KJ mol-1
Thus to break a particular C — H bond, the energy required is different than that required for another C — H bond in the same molecule. But the average C — H bond energy in methane = (435 + 443 + 443 + 343) /4 = 1664 /4 = 416 kJ
Heat of Reaction from Bond Enthalpy:
A chemical reaction involves breaking and forming of chemical bonds. During the formation of the bond, energy is released and during the breaking of a bond, energy is absorbed.
The enthalpy changes involving gaseous reactants and gaseous products having covalent bonds can be calculated with the help of bond enthalpies of reactants and products using following formula
Heat of Reaction = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
Numerical Problems on Bond Enthalpy:
Example – 01:
Calculate the enthalpy change of the reaction
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g),
ΔH°C-H = 414 KJ mol-1, ΔH°Cl-Cl = 243 KJ mol-1, ΔH°C-Cl = 330 KJ mol-1, ΔH°H-Cl = 431 KJ mol-1,
Solution:
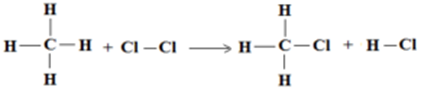
Heat of Reaction = ΔH° = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
∴ ΔH° = (4 × ΔH°C-H + 1 × ΔH°Cl-Cl ) – (3× ΔH°C-H + 1 × ΔH°C-Cl + 1 × ΔH°H-Cl )
∴ ΔH° = (4 mol × 414 KJ mol-1 + 1 mol × 243 KJ mol-1 ) – (3 mol × 414 KJ mol-1 + 1 mol × 330 KJ mol-1 + 1 mol × 431 KJ mol-1)
∴ ΔH° = (1656 KJ + 243 KJ) – (1242 kJ + 330 KJ + 431 KJ)
∴ ΔH° = 1899 KJ – 2003 kJ = – 104 kJ
Ans: Hence Reaction enthalpy is – 104 kJ
Example – 02:
Calculate the N-N bond enthalpy in the reaction
N2H4(g) + H2(g) → 2NH3(g) ΔH° = – 184 KJ
ΔH°N-H = 389 KJ mol-1, ΔH°H-H = 435 KJ mol-1,
Solution:
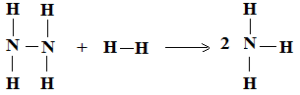
Heat of Reaction = ΔH° = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
∴ ΔH° = (4 × ΔH°N-H + 1 × ΔH°N-N + 1 × ΔH°H-H ) – 2× (3× ΔH°N-H )
∴ – 184 kJ = (4 mol × 389 KJ mol-1 + 1 mol × ΔH°N-N + 1 mol × 435 KJ mol-1) – 2× (3mol × 389 KJ mol-1 )
∴ – 184 kJ = 1556 KJ + 1 mol × ΔH°N-N + 435 KJ – 2334 KJ
∴ – 184 kJ = – 343 KJ + 1 mol × ΔH°N-N
∴ 1 mol × ΔH°N-N = -184 kJ + 343 kJ
∴ 1 mol × ΔH°N-N = 159 kJ
∴ ΔH°N-N = + 159 kJ mol-1
Ans: The N-N bond enthalpy in the reaction is 159 kJ mol-1
Example – 03:
Calculate the N-H bond enthalpy in the reaction
N2(g) + 3H2(g) → 2NH3(g) ΔH° = – 83 KJ
ΔH°N≡N = 946 KJ mol-1, ΔH°H-H = 435 KJ mol-1,
Solution:

Heat of Reaction = ΔH° = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
∴ ΔH° = (1 × ΔH°N≡N + 3 × ΔH°H-H ) – 2× (3× ΔH°N-H )
∴ – 83 kJ = (1 mol × 946 KJ mol-1 + 3 mol × 435 KJ mol-1) – 6 mol × ΔH°N-H
∴ – 83 kJ = 946 KJ + 1305 KJ – 6 mol × ΔH°N-H
∴ – 83 kJ = 2251 KJ – 6 mol × ΔH°N-H
∴ 6 mol × ΔH°N-H = 2251 KJ + 83 kJ
∴ 6 mol × ΔH°N-H = 2234 KJ
∴ ΔH°N-H = 2234 KJ / 6 mol
∴ ΔH°N-H = + 389 KJ mol-11
Ans: The N-H bond enthalpy in the reaction is 389 kJ mol-1
Example – 04:
Calculate the standard enthalpy ΔH° of the reaction
CH4(g) + O2(g) → CH2O(g) + H2O(g), D
ΔH°C-H = 414 KJ mol-1, ΔH°O=O = 499 KJ mol-1, ΔH°C=O = 745 KJ mol-1, ΔH°O-H = 464 KJ mol-1,
Solution:

Heat of Reaction = ΔH° = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
∴ ΔH° = (4 × ΔH°C-H + 1 × ΔH°O=O ) – (2× ΔH°C-H + 1× ΔH°C=O + 2× ΔH°O-H )
∴ ΔH° = (4 mol × 414 KJ mol-1+ 1 mol × 499 KJ mol-1 ) – (2 mol × 414 KJ mol-1+ 1 mol 745 KJ mol-1+ 2 mol × 464 KJ mol-1 )
∴ ΔH° = (1656 kJ + 499 kJ) – (828 kJ + 745 kJ+ 928 kJ)
∴ ΔH° = 2155 kJ – 2501 kJ = – 346 kJ
Ans: The standard enthalpy ΔH° of the reaction is – 346 kJ
Example – 05:
Calculate C-Cl bond enthalpy from the following data
CH3Cl(g) + Cl2(g) → CH2 Cl2(g) + HCl(g), DΔH° = – 104 kJ
ΔH°C-H = 414 KJ mol-1, ΔH°Cl-Cl = 243 KJ mol-1, ΔH°H-Cl = 431 KJ mol-1,
Solution:

Heat of Reaction = ΔH° = ∑ ΔH° (reactant bonds) – ∑ ΔH° (products bonds)
∴ ΔH° = (3 × ΔH°C-H + 1 × ΔH°C-Cl + 1 × ΔH°Cl-Cl ) – (2× ΔH°C-H + 2× ΔH°C-Cl + 1× ΔH°H-Cl )
∴ – 104 kJ= (3 mol × 414 KJ mol-1 + 1 mol × ΔH°C-Cl + 1 mol × 243 KJ mol-1 ) – (2 mol × 414 KJ mol-1+ 2 mol × ΔH°C-Cl1+ 1 mol × 431 KJ mol-1 )
∴ – 104 kJ= (1242 KJ + 1 mol × ΔH°C-Cl + 243 KJ) – (828 KJ + 2 mol × ΔH°C-Cl+ 431 KJ )
∴ – 104 kJ= (1485 KJ + 1 mol × ΔH°C-Cl ) – (1259 KJ + 2 mol × ΔH°C-Cl )
∴ – 104 kJ= 1485 KJ + 1 mol × ΔH°C-Cl – 1259 KJ – 2 mol × ΔH°C-Cl
∴ – 104 kJ= 226 KJ – 1 mol × ΔH°C-Cl
∴ 1 mol × ΔH°C-Cl = 226 KJ + 104 kJ = 330 kJ
∴ ΔH°C-Cl = 330 kJ mol-1
Ans: The C-Cl bond enthalpy is 330 kJ mol-1
Previous Topic: Change in Enthalpy of Different Processes
Next Topic: Hess’s Law and its Applications