Science > Chemistry > Chemical Thermodynamics and Energetics > Heat of Reaction Of Different Processes
In this article, we shall study change in enthalpy for different chemical processes.
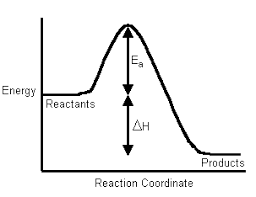
Enthalpy of Formation (ΔfH° or ΔformationH°):
The change in enthalpy of a chemical reaction at a given temperature and pressure, when one mole of the substance is formed from its constituent elements in their standard states is called the heat of formation.
Explanation: Consider the following reaction
C(s) + O2(g) → CO2(g) , ΔformationH° = -395.39 kJ mol-1
Since one mole of carbon dioxide gas is formed we can say that the heat of formation of carbon dioxide gas is -395.39 kJ.
Notes:
- The standard state of the element is that stable state of the element in which it exists at 1 atm. Pressure and 298 K
- Enthalpies of elements in their standard states are arbitrarily taken as zero.
- Enthalpy of a compound is equal to its heat of formation.
- When solving problems on the heat of formation make sure that the product side of the thermochemical equation has one mole of the substance whose heat of formation is to be calculated.
Enthalpy of Dissociation (ΔdissociationH°):
Change in enthalpy of a chemical reaction at a given temperature and pressure, when one mole of a substance is dissociated into its constituent elements is called the heat of dissociation.
Explanation: Consider the following reaction
H2(g) → 2H(g), ΔdissociationH° = + 435.136 kJ mol-1
Since one mole of hydrogen gas is dissociated the heat of dissociation of hydrogen gas is + 435.136 kJ
Enthalpy of Combustion (ΔcH° or ΔcombustionH°):
Change in the enthalpy of a chemical reaction at a given temperature and pressure when one mole of a substance is combusted (burn) completely in excess of oxygen is called the heat of combustion.
Explanation: Consider the following reaction
C(s) + O2(g) → CO2(g) , ΔH = -395.39 kJ mol-1
Since one mole of carbon is combusted completely the heat of combustion of carbon is – 395.39 kJ.
Enthalpy of Neutralization (ΔneutralizationH°):
Change in the enthalpy of a chemical reaction at a given temperature and pressure when one gram equivalent weight of acid is completely neutralized by one gram equivalent weight of the base is called the heat of neutralization.
Explanation: Consider following reaction
HCl(aq) + NaOH(aq) → NaCl + H2O ΔneutralizationH° = -56.9 kJ
The heat of neutralization of HCI by NaOH is -56.9 KJ.
For all strong acids and bases, the heat of neutralization is the same because, in their neutralization reaction, there is a combination of H+ ions of an acid with OH- ions of the base to produce un-dissociated water.
Change of Phase:
Change of phase involves the change in the physical state of matter. During the phase change, the chemical properties of the substance do not change but physical properties change. The following are the types of phase changes.
Fusion: This is the process in which the matter changes from solid-state to liquid state. It is endothermic process.
e.g. melting of ice H2O(s) → H2O(l)
Vapourization: This is the process in which the matter changes from liquid state to gaseous state. It is an endothermic process.
e.g. boiling of water H2O(l) → H2O(g)
Sublimation: This is the process in which the matter changes from the solid-state into a gaseous state directly. It is an endothermic process.
e.g. heating of camphor Camphor(s) → Camphor(g)
Characteristics of Change of Phase:
- The phase change always takes place at constant pressure and temperature.
- During the phase transition, there is an equilibrium between the two phases. Thus both the phases exist simultaneously.
- The Change in temperature takes place only when completion of phase transition.
Enthalpy of Fusion (ΔfusH°):
The enthalpy-change that accompanies the fusion of one mole of a solid without the change in temperature at constant pressure is called its enthalpy of fusion.
Explanation: Consider the following reaction
H2O(s) → H2O(l), ΔfusionH = +6.01 kJ mol-1
Thus, the equation indicates that when one mole of ice melts at 0° C at 1 atm pressure, the enthalpy-change is 6.01 kJ. i.e. 6.01 kJ of energy is absorbed.
Enthalpy of Freezing (ΔfreezeH°):
The enthalpy change that accompanies the freezing of one mole of a liquid without a change in temperature at constant pressure is called its enthalpy of freezing.
Explanation: Consider the following reaction
H2O(l) → H2O(s), ΔfreezeH = +6.01 kJ mol-1
Thus, the equation indicates that when one mole of water freezes at 0° C at 1 atm pressure, the change in enthalpy is -6.01 kJ. i.e. 6.01 kJ of energy is released.
Enthalpy of Vaporization (ΔvaporizationH°):
The enthalpy change that accompanies the vaporization of one mole of a liquid without a change in temperature at constant pressure is called its enthalpy of vaporization.
Explanation: Consider the following reaction
H2O(l) → H2O(g), ΔvapourizationH = + 40.7 kJ mol-1
Thus, the equation indicates that when one mole of water vaporizes at 100° C at 1 atm pressure, the change in enthalpy is + 40.7 kJ. i.e. 40.7 kJ of energy is absorbed.
Enthalpy of Condensation (ΔcondensationH°):
The enthalpy change that accompanies the condensation of one mole of a liquid without a change in temperature at constant pressure is called its enthalpy of condensation.
Explanation: Consider the following reaction
H2O(l) → H2O(g), ΔcondensationH = – 40.7 kJ mol-1
Thus, the equation indicates that when one mole of water vapours condense at 100° C at 1 atm pressure, the enthalpy-change is – 40.7 kJ. i.e. 40.7 kJ of energy is released.
Enthalpy of Sublimation (ΔsublimationH°):
The direct conversion of solid to vapour without going through the liquid state is called sublimation. The enthalpy-change that accompanies the condensation of one mole of a solid directly into vapours at a constant temperature at constant pressure is called its enthalpy of sublimation.
Explanation: Consider the following reaction
H2O(s) → H2O(g), ΔsublimationH = +51.08 kJ mol-1
Thus, the equation indicates that when one mole of ice sublimes at O° C at 1 atm pressure, the enthalpy change is + 51.08 kJ. i.e. 51.8 kJ of energy is absorbed.
It is to be noted that ΔsublimationH = ΔfusionH + ΔvapourizationH
Atomic or Molecular Changes:
Enthalpy of Ionization (ΔionizationH°):
The enthalpy change that accompanies the removal of an electron from each atom or ion in one mole of gaseous atoms or ions is called its enthalpy of ionization.
Explanation: Consider the following reaction
Na(g) → Na+(g)+ e– , ΔionizationH = 494 kJ mol-1
Thus, the equation indicates that when one mole of gaseous sodium atom ionizes to Na+(g) ion, the change in enthalpy is + 494 kJ. i.e. 494 kJ of energy is absorbed.
Enthalpy of Atomization (ΔatomizationH°):
The enthalpy change that accompanies the dissociation of all the molecules in one mole of gas-phase substance into gaseous atoms is called its enthalpy of atomization.
Explanation: Consider the following reaction
Cl2(g) → Cl(g)+ Cl(g), ΔatomizationH = 242 kJ mol-1
Thus, the equation indicates that when one mole of gaseous chlorine molecule dissociates completely into its atomic form in the gaseous state then the change in enthalpy is + 242 kJ. i.e. 242 kJ of energy is absorbed.
Enthalpy of Solution (ΔsolutionH°):
Change in enthalpy of chemical reaction at a given temperature and pressure, when one mole of a solution is dissolved in a specified quantity of solvent so as to form a solution of particular concentration is called as enthalpy of a solution.
Explanation: Consider the following reaction
KOH(s) + H2O(l) → KOH(aq) ΔsolutionH = -58.57 KJ mol-1
Thus, the equation indicates that when one mole of potassium hydroxide (solute) dissolves in one mole of water (solvent) to form one mole of potassium hydroxide solution in water then the change in enthalpy is -58.57 kJ. i.e. 58.57 kJ of energy is released
Bond Enthalpy (Bond Energy):
Chemical reactions involve the breaking and making of chemical bonds. Energy is required to break a bond and energy is released when a bond is formed. It is possible to relate the heat of reaction to changes in energy associated with the breaking and making of chemical bonds. With reference to the enthalpy changes associated with chemical bonds, two different terms are used in thermodynamics. (i) Bond dissociation enthalpy (ii) Mean bond enthalpy. Let us discuss these terms with reference to diatomic and polyatomic molecules.
Diatomic Molecules:
Consider the following process in which the bonds in one mole of dihydrogen gas (H2) are broken:
H2(g) → 2H(g) ; ΔH–HHO = 435.0 kJ mol-1
The enthalpy change involved in this process is the bond dissociation enthalpy of H–H bond.
The bond dissociation enthalpy is the change in enthalpy when one mole of covalent bonds of a gaseous covalent compound is broken to form products in the gas phase. Note that it is the same as the enthalpy of atomization of dihydrogen. This is true for all diatomic molecules.
Polyatomic Molecule:
In the case of polyatomic molecules, bond dissociation enthalpy is different for different bonds within the same molecule. Let us consider a polyatomic molecule like methane, CH4. The overall thermochemical equation for its atomization reaction is given below:
CH4(g) → C(g) + 4H(g) , ΔH = +1665 KJ.
In methane, all the four C – H bonds are identical in bond length and energy. However, the energies required to break the individual C – H bonds in each successive step differ. In such cases, we use mean bond enthalpy
CH4(g) → CH3(g) + H(g) , ΔH = +427 KJ.
CH3(g) → CH2(g) + H(g), ΔH = +439 KJ
CH2(g) → CH(g) + H(g), ΔH = +452 KJ
CH(g) → C (g) + H(g), ΔH = +347 KJ
Adding above reactions we get
CH4(g) → C(g) + 4H(g) , ΔH = +1665 KJ
We find that mean C–H bond enthalpy in methane as 1664/4 = 416 kJ/mol. Using Hess’s law, bond enthalpies can be calculated.
Enthalpy of Reaction from Bond Enthalpy:
The reaction enthalpies are very important quantities as these arise from the changes that accompany the breaking of old bonds and the formation of the new bonds. We can predict the enthalpy of a reaction in the gas phase if we know different bond enthalpies. The standard enthalpy of reaction is related to bond enthalpies of the reactants and products in gas phase reactions as:
ΔH = ∑ Bond enthalpies reactants – ∑ Bond enthalpies products
Remember that this relationship is approximate and is valid when all substances (reactants and products) in the reaction are in a gaseous state.
The values of given bond enthalpy can be used to calculate bond enthalpies of specific bonds in the molecule.
Crystal Lattice Energy (ΔLatticeH°):
Crystal lattice energy is defined as the enthalpy change or energy released accompanying formation of 1 mole of crystalline solid from its constituent ions in the gaseous state at a constant temperature.
Explanation:
M+(g) + X–(g) → M+X–(s) , ΔH = – x KJ mol-1
Thus, the equation indicates that when one mole of ionic compound M+X-(s) is formed from its constituent ions the change in enthalpy is – x kJ i.e. x kJ of energy is evolved. Crystal lattice energy is always negative.
The sequence of actions involved in the formation of 1 mole of an ionic compound in its standard state from its constituent elements in their states at constant temperature and pressure is called Born-Haber cycle.
Factors Affecting Crystal Lattice Energy:
Crystal lattice energy depends on the interionic distance in the crystalline solid. As the distance decreases, the crystal lattice energy increases. Crystal lattice energy depends on the charge of constituent cations and anions.
Enthalpy of Hydration (ΔHydrationH°):
It is defined as the heat evolved when one mole of gaseous ions dissolve in water by hydration to give infinitely dilute solution at constant temperature and pressure.
Explanation: Consider the following reaction
Na+(g) + aq → Na+(ag) , ΔH = – 390 KJ mol-1
Thus, the equation indicates that when one mole of sodium ion in the gaseous state is dissolved in water the change in enthalpy is – 390 kJ. i.e. 390 kJ of energy is released.
Previous Topic: Enthalpy of Reaction