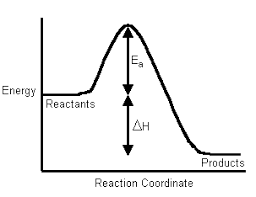
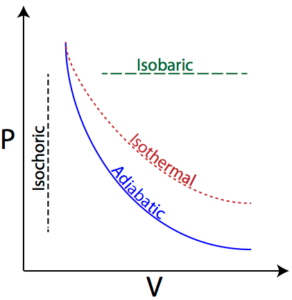
Science > Chemistry > Chemical Thermodynamics and Energetics > Change in Internal Energy and Enthalpy In this article, we shall study to calculate the change in internal energy and change in enthalpy in a chemical reaction. Example – 01: For a particular reaction, the system absorbs 6 kJ of heat and does 1.5 kJ of […]