Science > Chemistry > Chemical Thermodynamics and Energetics > Concept of Maximum Work
According to the first law of thermodynamics, ΔU = q + W In an isothermal process, ΔU = 0, ∴ q = – W
Therefore, all the heat absorbed by the system is utilized to do work. (i.e. maximum utilization of the energy takes place. )
The work of expansion is given by the product of external pressure and the volume change. W = – Pext ΔV
In any expansion, the external pressure must be less than the pressure of the gas. If the external pressure is zero, the work done is also zero as the gas expands into the vacuum. If the external pressure is increased gradually, more and more work will be done by the gas during expansion. If the external pressure becomes equal to the pressure of the gas, there will be no change in the volume and thus ΔV = 0. The work done is also zero. If Pext is more than the pressure of the gas cannot expand. Therefore, when Pext becomes P then ΔV will be maximum. In the reversible process, Pext is always less than the pressure of the gas, by an infinitesimally small quantity.
W = (P – dp) dV
In the equation W tends to the maximum as (P – dp) tends to P or dp tends to zero. Therefore work done in an isothermal reversible expansion of an ideal gas is maximum work.
Conditions for Maximum Work:
All the changes taking place in a system during the process are reversible. All the changes taking place in a system during the process should take place in infinitesimally small infinite steps. During the change, the driving and opposing forces should defer by an infinitesimally small amount. The system remains in mechanical equilibrium with the surroundings.
Expression for Maximum Work:
Work done in an isothermal reversible expansion is maximum work.
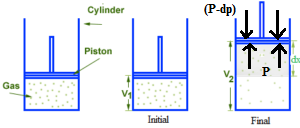
Consider ‘n ‘ moles of an ideal gas enclosed in a cylinder fitted with a weightless, frictionless, airtight movable piston. Let the pressure of the gas be P which is equal to external atmospheric pressure P. Let the external pressure be reduced by an infinitesimally small amount dp and the corresponding small increase in volume be dV. So the small work done in the expansion process
dW = – Pext . dV
∴ dW = – (P – dp). dV
∴ dW = – (P.dV – dp.dV) ….. (1)
Since both dp and dV are very small, the product dp.dV is very small and can be neglected in comparison with P.dV. Then the above equation becomes
dW = – P.dV ………. (2)
When the expansion of the gas is carried out reversibly then there will be series of such p.dV terms. The total maximum work Wmax can be obtained by integrating above equation between the limits V1 to V2. Where V1 is initial volume and V2 is final volume.
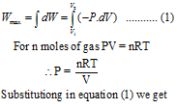
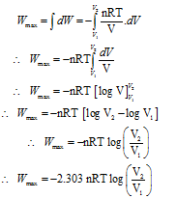
For an isothermal expansion, Boyle’s law is applicable.
Hence P1V1 = P2V2
i.e. V2 / V1 = P1 / P2

Where P1 and P2 are initial and final pressure respectively.
Notes:
- As the ratio of volumes or pressure is used in the above equation when work done is to be calculated the volumes and the pressures may be expressed in any unit, provided both the quantities are expressed in the same unit.
- The work obtained using the above equation will be in joule if R is taken in the S.I. unit.
- The number of moles = Wt. in gram / Molecular wt.in grams
- Absolute temperature T k = to C + 273
Work is Path Function:
The work done in isothermal constant pressure process is given by
W = – Pext ΔV i.e. W = – Pext ( V2 – V1)
Where, Pext = External opposing pressure
V1 = Initial volume V2 = Final volume.
The work done in the isothermal reversible process is given by

Where, n = Number of moles of the gas R = Universal gas constant
T = Absolute temperature of the gas
V1 = Initial volume V2 = Final volume
We can see that the work done depends on the manner or the conditions under which the process carried out. Thus it is not dependent on initial and final conditions of the system but on the path followed by the system. Hence work is path function or it is not a state function.
Numerical Problems on Maximum Work:
Example – 01:
3 moles of an ideal gas are expanded isothermally and reversibly from volume of 10 m3 to the volume 20 m3 at 300 K. Calculate the work done.
Given: n = 3 moles, V1 = 10 m3, V2 = 20 m3, T = 300 K, R = 8.314 J K-1 mol-1.
To Find: Work done =?
Solution:
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(V2/V1)
∴ Wmax = -2.303 × 3 mol × 8.314 J K-1 mol-1 × 300 K × log10( 20 m3/ 10 m3)
∴ Wmax = -2.303 × 3 × 8.314 × 300 × log10(2)
∴ Wmax = -2.303 × 3 × 8.314 × 300 × 0.3010
∴ Wmax = – 5187 J = – 5.187 kJ
Ans: maximum work done = – 5187 J = – 5.187 kJ
Negative sign indicates the work is done by the system on the surroundings
Example – 02:
24 g of oxygen are expanded isothermally and reversibly from 1.6 × 105 Pa pressure to 100 kPa at 298 K. Calculate the work done.
Given: m = 24 g, P1 = 1.6 × 105 Pa, P2 = 100 kPa = 100 × 103 Pa = 1 × 105 Pa, T = 298 K, R = 8.314 J K-1 mol-1.
To Find: Work done =?
Solution:
Number of moles of oxygen = Given mass of oxygen / molecular mass of oxygen
Number of moles of oxygen = 24 g / 32 g mol-1 = 0.75 mol
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(P1/P2)
∴ Wmax = -2.303 × 0.75 mol × 8.314 J K-1 mol-1 × 298 K × log10( 1.6 × 105 Pa / 1 × 105 Pa)
∴ Wmax = -2.303 × 0.75 × 8.314× 298 × log10( 1.6)
∴ Wmax = -2.303 × 0.75 × 8.314 × 298 × 0.2041
∴ Wmax = – 873.4 J
Ans: maximum work done = – 873.4 J
Negative sign indicates the work is done by the system on the surroundings
Example – 03:
4.4 × 10-2 kg of CO2 is compressed isothermally and reversibly at 293 K from the initial pressure of 150 kPa when work obtained is 1.245 kJ. Find the final pressure.
Given: m = 4.4 × 10-2 kg, P1 = 150 kPa, Maximum work of compression = W = + 1.245 kJ = 1245 J, T = 293 K, R = 8.314 J K-1 mol-1, P2 =?
To Find: Final pressure =?
Solution:
Number of moles of CO2 = Given mass of CO2 / molecular mass of CO2
Number of moles of CO2 = n = 4.4 × 10-2 kg / 44 × 10-3 kg mol-1 = 1 mol
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(P1/P2)
∴ 1245 J = -2.303 × 1 mol × 8.314 J K-1 mol-1 × 293 K × log10(P1/P2)
∴ 1245 J = -2.303 × 1 mol × 8.314 J K-1 mol-1 × 293 K × log10(P2/P1)
∴ log10(P2/P1) = (1245) J / (2.303 × 1 × 8.314 × 293) J
∴ log10(P2/P1) = 0.2219
∴ (P2/P1) = Antilog (0.2219) = 1.667
∴ P2 = 1.667 × P1 = 1.667 × 150 kPa = 250 kPa
Ans: Final Pressure is 250 kPa
Example – 04:
2.8 × 10-2 kg of nitrogen is expanded isothermally and reversibly at 300 K from the initial pressure of 15.15 × 105 Nm-2 when work obtained is 17.33 kJ. Find the final pressure.
Given: m = 42.8 × 10-2 kg, P1 = 15.15 × 105 Nm-2, Maximum work of expansion = W = – 17.33 kJ = – 17.33 × 103 J, T = 293 K, R = 8.314 J K-1 mol-1, P2 =?
To Find: Final Pressure =?
Solution:
Number of moles of Nitrogen = Given mass of Nitrogen / molecular mass of Nitrogen
Number of moles of Nitrogen = n = 2.8 × 10-2 kg / 28 kg mol-1 = 1 mol
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(P1/P2)
∴ – 17.33 × 103 J = -2.303 × 1 mol × 8.314 J K-1 mol-1 × 300 K × log10(P1/P2)
∴ 17.33 × 103 J = 2.303 × 1 mol × 8.314 J K-1 mol-1 × 300 K × log10(P1/P2)
∴ log10(P1/P2) = (17.33 × 103) J / (2.303 × 1 × 8.314 × 300) J
∴ log10(P1/P2) = 3.0170
∴ (P1/P2) = Antilog (3.0170) = 1040
∴ P2 = P1 / 1040 = 15.15 × 105 Nm-2 / 1040 = 14056.7 Nm-2
Ans: Final Pressure is 14056.7 Nm-2
Example – 05:
3 moles of an ideal gas are compressed isothermally and reversibly at 22° C to a volume 2L when work done is 2.983 kJ. Find the initial volume.
Given: n = 3 mol, V2 = 2 L, Maximum work of compression = W = + 2.983 kJ = 2.983 × 103 J , T = 22° C = 22 + 273 = 295 K, R = 8.314 J K-1 mol-1, V1 = ?,
To Find: Initial volume =?
Solution:
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(V2/V1)
∴ 2.983 × 103 J = -2.303 ×3 mol × 8.314 J K-1 mol-1 × 295 K × log10(V2/V1)
∴ 2.983 × 103 J = 2.303 ×3 mol × 8.314 J K-1 mol-1 × 295 K × log10(V1/V2)
∴ log10(V1/V2) = 2.983 × 103 J / (2.303 × 3 × 8.314 × 295) J
∴ log10(V1/V2) = 0.1760
∴ (V1/V2) = Antilog (0.1760) = 1.5
∴ V1= 1.5 × V2 = 1.5 × 2 L = 4 L
Ans: Initial Volume = 4 L
Example – 06:
280 mmol of an ideal gas occupy 12.7 L at 310 K. Calculate the work done when gas expands a) isothermally against constant external pressure 0.25 atm, b) isothermally and reversibly c) in a vacuum until its volume becomes 3.3 L.
Given: n = 280 mmoles = 0.280 mol, V1 = 12.7 L, ΔV = 3.3 L, T = 310 K, R = 8.314 J K-1 mol-1. External Pressure = 0.25 atm
To Find: Work done =?
Solution:
a) The gas expands isothermally against constant external pressure 0.25 atm
W = – Pext × ΔV = – 0.25 atm × 3.3 L = – 0.825 L atm
W = – 0.825 L atm × 101.3 J L-1 atm-1 = – 83.6 J
Hence work done = – 83.6 J
Negative sign indicates the work is done by the system on the surroundings
b) Gas expands isothermally and reversibly
ΔV = (V2 – V1) = (V2 – 12.7 L) = 3.3 L
V2 = 3.3 L + 12.7 L = 16 L
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(V2/V1)
∴ Wmax = -2.303 × 0.280 mol × 8.314 J K-1 mol-1 × 310 K × log10( 16 L/ 12.7 L)
∴ Wmax = -2.303 × 0.280 × 8.314 × 310 × log10(1.260)
∴ Wmax = -2.303 × 0.280 × 8.314 × 310 × 0.1004
∴ Wmax = – 166.9 J
Maximum work = 166.9 J
Negative sign indicates the work is done by the system on the surroundings
c) Gas expands in vacuum
In this case, it is a free expansion, there is no opposing pressure. Hence Pext = 0
W = – Pext × ΔV = 0 atm × 3.3 L = 0
Work done = 0
Example – 07:
300 mmol of a perfect gas occupies 13 L at 320 K. Calculate the work done in joules when the gas expands a) isothermally against the constant external pressure of 0.20 atm. b) Isothermal and reversible process c) Into vacuum until the volume of the gas is increased by 3 L.
Given: n = 300 mmoles = 0.3 mol, V1 = 13 L, ΔV = 3 L, T = 320 K, R = 8.314 J K-1 mol-1. External Pressure = 0.20 atm
To Find: Work done =?
Solution:
a) The gas expands isothermally against constant external pressure 0.20 atm
W = – Pext × ΔV = – 0.20 atm × 3 L = – 0.6 L atm
W = – 0.6 L atm × 101.3 J L-1 atm-1 = – 60.78 J
Hence work done = – 60.78 J
Negative sign indicates the work is done by the system on the surroundings
b) Gas expands isothermally and reversibly
ΔV = (V2 – V1) = (V2 – 13 L) = 3 L
V2 = 3 L + 13 L = 16 L
Work done in isothermal reversible process is given by
Wmax = -2.303 nRT log10(V2/V1)
∴ Wmax = -2.303 × 0.3 mol × 8.314 J K-1 mol-1 × 320 K × log10( 16 L/ 13 L)
∴ Wmax = -2.303 × 0.3 × 8.314 × 320 × log10(1.231)
∴ Wmax = -2.303 × 0.3 × 8.314 × 320 × 0.0902
∴ Wmax = – 165.8 J
Maximum work = – 165.8 J
Negative sign indicates the work is done by the system on the surroundings
c) Gas expands in vacuum
In this case, it is the free expansion, there is no opposing pressure. Hence Pext = 0
W = – Pext × ΔV = 0 atm × 3 L = 0
Work done = 0
Previous Topic: Pressure-Volume Type Work
Next Topic: First Law of Thermodynamics
3 replies on “Concept of Maximum Work”
Very helpful for jee preparation
Very useful
Good