Science > Chemistry > Chemical Thermodynamics and Energetics > Work Done in Chemical Process
Pressure Volume Work:
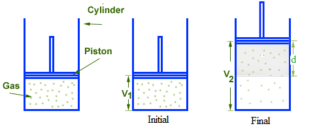
Consider an ideal gas having definite mass (say n moles) be enclosed in a cylinder fitted with weightless, frictionless, tightly fitted, movable piston. Let ‘A’ be the area of the cross-section of the cylinder. Let the gas expand from volume V1 to V2 against constant external pressure Pext which is exerted on the piston. Due to expansion, the piston moves upward by a distance ‘d’. In this process, the system loses energy to the surroundings. Hence the work done by the system is negative.
Work done = – Opposing Force x displacement
∴ W = – F x displacement ……. (1)
Now, pressure is force per unit area.
∴ Pressure = Force / Area
∴ Force = Pressure x Area
∴ F = P x A …….. (2)
Substituting this value in equation (1)
∴ W = – P x A x dx ………. (3)
But, A x dx = Volume through gas expands
= Change in volume = ( V2 – V1) = Δ V
Substituting this value in equation (3)
W = – Pext ΔV
This is an expression for pressure volume work done in terms of pressure and volume in the isothermal expansion of an ideal gas against constant external pressure.
Notes:
- It is the external pressure against gas expands i.e. Pext and not the pressure of the gas that is used in evaluating the pressure volume work done.
- The final equation shows that work W depends only on the change in volume (ΔV) and opposing pressure (Pext) and not on the quantity of the gas (i.e. it is independent of the number of moles) and Temperature of the gas (T).
- The work done by the system in a process depends on the way or manner in which it is carried out. Work, therefore, is not a state function. It is a path function.
Free Expansion:
The free expansion is also known as work of expansion in a vacuum. When a gas expands in a vacuum there is no opposing force i.e. Pext = 0.
For getting work done opposing force is necessary, hence no work is done when a gas expands in a vacuum. Such an expansion is called as free expansion.
W = – Pext ΔV
∴ W = 0 × ΔV = 0
Work Done in a Cyclic Process:
A cyclic process is one which consists of a series of intermediate steps, at the end of which the system returns to its initial state.
Since in a cycle, initial and final states are the same.
∴ Δ(state function) = 0
∴ ΔU = 0 (i.e. No change in internal energy)
Now, ΔU = q + W
∴ 0 = q + W
∴ q = -W
Thus in a cyclic process heat absorbed by the system from the surrounding (q) is equal to work done (W) by the system on the surrounding.
Difference Between Heat and Work:
When heat is added to gas the random motion of molecules of gas increases, thus heat is a stimulus to gas which increases the random motion of the gas molecules. Thus heat is a random form of energy. Now let us consider work done on a system by compressing the gas in a cylinder. The movement of the piston makes the gas molecules to move in the direction of applied force. Thus work is a stimulus which increases the organized motion of the molecules of the gas. Thus work is an organized form of energy.
Sign Convention Used in Thermodynamics:
- During the expansion, V2 > V1, Hence ΔV is positive. Hence work done W is negative. Thus when the work is done by the system on the surrounding i.e. Work of expansion is taken as negative (- W).
- During compression, V2 < V1, Hence ΔV is negative. Hence work done W is positive. Thus when the work done on the system by the surrounding i.e. Work of compression is taken as positive (+W).
Numerical Problems on Pressure Volume Work:
Example – 01:
2 moles of an ideal gas are expanded isothermally from volume of 15.5 L to the volume 20 L against a constant external pressure of 1 atm. Calculate the pressure-volume work in L atm and J.
Given: n = 2 moles, V1 = 15.5 L, V2 = 20 L, Pext = 1 atm
To Find: Work Done =?
Solution:
Work done in an isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 1 atm × (20 L – 15.5 L)
∴ W = – 1 atm × (4.5 L) = – 4.5 L atm
W = – 4.5 L atm × 101.3 J L-1 atm-1 = – 455.8 J
Ans: work done = – 4.5 L atm or – 455.8 J
The negative sign indicates the work is done by the system on the surroundings
Example – 02:
2 moles of an ideal gas are compressed isothermally from volume of 10 dm3 to the volume 2 dm3 against a constant external pressure of 1.01 × 105 Nm-2. Calculate the pressure-volume work done.
Given: n = 2 moles, V1 = 10 dm3 = 10 × 10-3 m3, V2 = 2 dm3 = 2 × 10-3 m3, Pext = 1 atm.
To Find: Work Done =?
Solution:
Work done in an isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 1.01 × 105 Nm-2 × (2 × 10-3 m3 – 10 × 10-3 m3)
∴ W = – Pext × (V2 – V1) = – 1.01 × 105 Nm-2 × (- 8 × 10-3 m3)
∴ W = + 8.08 × 102 J = + 808 J
Ans: work done = + 808 J
Positive sign indicates the work is done by the surrounding on the system
Example – 03:
3 moles of an ideal gas are expanded isothermally from volume of 300 cm3 to the volume 2.5 L against a constant external pressure of 1.9 atm at 300 K. Calculate the pressure volume work in L atm and J.
Given: n = 3 moles, V1 = 300 cm3= 0.3 L, V2 = 2.5 L, Pext = 1.9 atm
To Find: Work Done =?
Solution:
Work done in an isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 1.9 atm × (2.5 L – 0.3 L)
∴ W = – 1.9 atm × (2.2 L) = – 4.18 L atm
W = – 4.18 L atm × 101.3 J L-1 atm-1 = – 423.4 J
Ans: work done = – 4.5 L atm or – 423.4 J
The negative sign indicates the work is done by the system on the surroundings
Example – 04:
1 mole of an ideal gas is compressed isothermally from a volume of 500 cm3 against a constant external pressure of 1.216 × 105 Pa. The pressure-volume work involved in the process is 36.5 J. calculate the final volume.
Given: n = 1 mole, V1 = 500 cm3= 500 × 10-6 m3, Pext = 1.216 × 105 Pa = = 1.216 × 105 Nm-2, Work of compression = + 36. 5 J, V2 =?
To Find: Final volume = V2 =?
Solution:
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1)
∴ 36.5 J= – 1.216 × 105 Nm-2 × (V2 – 500 × 10-6 m3)
∴ 36.5 J/ 1.216 × 105 Nm-2 = – (V2 – 500 × 10-6 m3)
∴300 × 10-6 m3 = (500 × 10-6 m3 – V2)
∴ V2 = (500 × 10-6 m3 – 300 × 10-6 m3)
∴ V2 = 200 × 10-6 m3 = 200 cm3
Ans: final volume = 200 cm3
Example – 05:
1 mole of an ideal gas is compressed isothermally from volume of 20 L to 8 L against constant external pressure, when pressure volume work obtained is 44.9 L atm. Find the constant external pressure.
Given: n = 1 mol, V1 = 20 L, V2 = 8 L, Work of compression W = + 44.9 L atm, Pext =?
To Find: External pressure =?
Solution:
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1)
∴ 44.9 L atm = – Pext × (8 L – 20 L)
∴ 44.9 L atm = – Pext × (- 12 L)
∴ Pext =44.9 L atm / 12 L)
∴ Pext = 3.74 atm
Ans: constant external pressure is 3.74 atm
Example – 06:
One mole of a gas expands by 3 L against a constant pressure of 3 atmosphere. Calculate the pressure volume work done in a) L-atm b) joules and c) calories.
Given: n = 1 mole, Δ V = 3 L, Pext = 3 atm
To Find: Work Done =?
Solution:
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – 3 atm × 3 L = – 9 L atm
∴ W = – 9 L atm × 101.3 J L-1 atm-1 = – 911.7 J
∴ W = – 911.7 J / 4.184 J cal-1 = – 217.9 cal
Ans: work done = – 9 L atm or – 911.7 J or -217.9 cal
Negative sign indicates the work is done by the system on the surroundings
Example – 07:
100 mL of ethylene(g) and 100 mL of HCl (g) are allowed to react at 2 atm pressure as per the reaction given below. Calculate pressure volume type of work in it in joules.
C2H4(g) + HCl(g) → C2H5Cl(g)
Solution:
Given: Pext = 2 atm and the given reaction is
C2H4(g) + HCl(g) → C2H5Cl(g)
1 Vol 1Vol 1Vol
100 mL 100 mL 100 mL
Thus 100 ml of C2H4(g) reacts with 100 mL of HCl(g) to give 100 mL of C2H5Cl(g).
Initial volume = Volume of reactants = 100 mL + 100 mL = 200 mL = 0.2 L
Final volume = Volume of products = 100 mL = 0.1 L
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 2 atm × (0.1 L – 0.2 L)
∴ W = – 2 atm × (- 0.1 L) = 0.2 L atm
W = 0.2 L atm × 101.3 J L-1 atm-1 = 20.26 J
Ans: work done = + 20.26 J
Positive sign indicates the work is done by the surroundings on the system
Example – 08:
A gas cylinder of 5 L capacity containing 4 kg of helium gas at 27 °C developed a leakage leading to the escape of the gas into atmosphere. If atmospheric pressure is 1.0 atm. calculate the pressure volume work done by the gas assuming ideal behaviour.
Given: V1 = 5 L, mass of gas m = 4 kg = 4 × 103 g, T = 27 °C = 27 +273 = 300 K, Pext = P = 1.0 atm, R = 0.0821 L atm K-1 mol-1.
To Find: Work Done =?
Solution:
Number of moles = Given mass of He / Molecular mass of He = 4 × 103 g / 4 g = 103
By ideal gas equation, we have P V2 = nRT
∴ V2 = nRT / P = 103 × 0.0821 × 300 / 1 = 2.463 × 104 L = 24630 L
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 1 atm × (24630 L – 5 L)
∴ W = – 1 atm × (24625 L) = – 24625 L atm
W = – 24625 L atm × 101.3 J L-1 atm-1 = – 2.494 × 106 J = – 2494 kJ
Ans: work done = – 2494 kJ
Negative sign indicates the work is done by the system on the surroundings
Example – 09:
1.6 mol of water evaporates at 373 K against atmospheric pressure of 1 atm. Assuming ideal behaviour of water vapours calculate the work done.
Given: n = 1.6 mole, T = 373 K, Pext = P = 1.0 atm, R = 0.0821 L atm K-1 mol-1.
To Find: Work Done =?
Solution:
The molecular mass of water 18 g. and the density of water is 1 g per cc
Hence initial volume of water = V1 = 18 x 1.6 x 10-3 L = 0.0288 L
By ideal gas equation, we have P V2 = nRT
∴ V2 = nRT / P = 1.6 × 0.0821 × 373 / 1 = 48.93 L
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 1 atm × (48.93 L – 0.0288 L)
∴ W = – 1 atm × (48.90 L) = – 48.90 L atm
W = – 48.90 L atm × 101.3 J L-1 atm-1 = – 4954.8 J
Hence work done = – 4954.8 J
Negative sign indicates the work is done by the system on the surroundings
Example – 10:
1.0 mol of water evaporates at 373 K against atmospheric pressure of 749.8 mm of Hg. Assuming ideal behaviour of water vapours calculate the pressure volume work done.
Given: n = 1.0 mole, T = 373 K, Pext = P = 749.8 mm of H = 749.8 / 760 = 0.987 atm, R = 0.0821 L atm K-1 mol-1.
To Find: Work Done =?
Solution:
The molecular mass of water 18 g. and the density of water is 1 g per cc
Hence initial volume of water = V1 = 18 x 1.0 x 10-3 L = 0.018 L
By ideal gas equation, we have P V2 = nRT
∴ V2 = nRT / P = 1.0 × 0.0821 × 373 / 0.987 = 31.03 L
Work done in isothermal process is given by W = – Pext × ΔV
∴ W = – Pext × (V2 – V1) = – 0.987 atm × (31.03 L – 0.018 L)
∴ W = – 0.987 atm × (31.01 L) = – 30.61 L atm
W = – 30.61 L atm × 101.3 J L-1 atm-1 = – 3101 J
Ans: work done = – 3101 J
Negative sign indicates the work is done by the system on the surroundings
Previous Topic: Concept of Internal Energy
Next Topic: Concept of Maximum Work
One reply on “Work Done in Chemical Process”
very helpful .thankyou