Science > Chemistry > Molecule and Molecular Mass > Calculation of Mass of a Molecule and an Atom
In this article, we shall study the calculation of the mass of molecule and an atom.
Schematic Diagram for Mole Calculations:
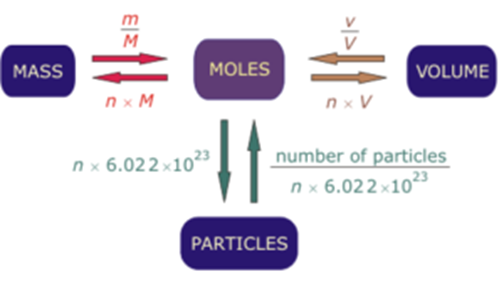
Where, m = Given mass, M = Molar mass
v = Given volume, V = Molar volume = 22.4 dm3
n = Number of moles = m/M
Number of atoms = Number of molecules × Atomicity
Conversions:
| From | To | Factor |
| kg | g | × 103 |
| mg | g | × 10-3 |
| μg | g | × 10-6 |
| metric ton | kg | × 103 |
| metric ton | g | × 106 |
| cm3 | dm3 | × 10-3 |
| m3 | dm3 | × 103 |
| litre | dm3 | × 1 |
To Calculate Mass of Given Moles:
Calculate the mass of the following.
2.5 moles of water:
Molecular mass of water (H2O) = 1 × 2 + 16 × 1 = 2 + 16 = 18 g
Number of moles of water = 2.5 = n
Mass of water = n × Molar mass
Mass of water = 2.5 × 18 = 45 g
1.2 moles of carbon dioxide
Molecular mass of carbon dioxide (CO2) = 12 × 1 + 16 × 2 = 12 + 32 = 44 g
Number of moles of carbon dioxide = 1.2
Mass of carbon dioxide = n × Molar mass
Mass of carbon dioxide = 1.2 × 44 = 52.8 g
0.25 moles of sulphuric acid
Molecular mass of sulphuric acid (H2SO4) = 1 x 2 + 32 x 1 + 16 x 4 = 2 + 32 + 64 = 98 g
Number of moles of sulphuric acid = 0.25 = n
Mass of sulphuric acid = n × Molar mass
Mass of sulphuric acid = 0.25 × 98 = 24.5 g
0.1 moles of ammonia
Molecular mass of ammonia (NH3) = 14 x 1 + 1 x 3 = 14 + 3 = 17 g
Number of moles of ammonia = 0.1 = n
Mass of ammonia = n × Molar mass
Mass of ammonia = 0.1 × 17 = 1.7 g
3.5 moles of methane
Molecular mass of methane (CH4) = 12 x 1 + 1 x 4 = 12 + 4 = 16 g
Number of moles of methane = 3.5 = n
Mass of methane = n × Molar mass
Mass of methane = 3.5 × 16 = 56 g
2.4 moles of sulphur dioxide
Molecular mass of sulphur dioxide (SO2) = 32 x 1 + 16 x 2 = 32 + 32 = 64 g
Number of moles of sulphur dioxide = 2.4 = n
Mass of sulphur dioxide = n × Molar mass
Mass of sulphur dioxide = 2.4 × 64 = 153.6 g
0.6 moles of bromine
Molecular mass of bromine (Br2) = 40 x 2 = 80 g
Number of moles of bromine = 0.6 = n
Mass of bromine = n × Molar mass
Mass of bromine = 0.6 × 80 = 48 g
To Calculate Mass of a Molecule and Atom:
Calculate the following
mass of one oxygen atom and oxygen molecule in kg.
Molecular mass of oxygen (O2) = 16 x 2 = 32 g
1 mole of oxygen is 32 g = 32 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of oxygen = (32 x 10-3)/(6.022 x 1023)
= 5.314 x 10-26 kg
Atomicity of oxygen (O2) molecule is 2
Mass of each atom of oxygen = 5.314 x 10-26 /2 = 2.657 x 10-26 kg
mass of one calcium atom in kg.
Molecular mass of calcium (Ca) = 40 g
1 mole of calcium is 40 g = 40 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of calcium = (40 x 10-3)/(6.022 x 1023)
= 6.642 x 10-26 kg
Atomicity of calcium (Ca) molecule is 1
Mass of each atom of calcium = 6.642 x 10-26 /1 = 6.642 x 10-26 kg
mass of one nitrogen atom and nitrogen molecule in kg.
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g
1 mole of nitrogen is 28 g = 28 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of nitrogen = (28 x 10-3)/(6.022 x 1023)
= 4.650 x 10-26 kg
Atomicity of nitrogen (N2) molecule is 2
Mass of each atom of nitrogen = 4.650 x 10-26 /2 = 2.325 x 10-26 kg
mass of one sulphur dioxide molecule in grams.
Molecular mass of sulphur dioxide (SO2) = 32 x 1 + 16 x 2 = 64 g
1 mole of sulphur dioxide is 64 g
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of sulphur dioxide = (64)/(6.022 x 1023)
= 1.602 x 10-22 kg
mass of 100 molecules of water.
Molecular mass of water (H2O) = 1 x 2 + 16 x 1 = 18 g
1 mole of water is 18 g = 18 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of water = (18 x 10-3)/(6.022 x 1023) = 2.989 x 10-26 kg
Mass of 100 molecules of water = 2.989 x 10-26 x 100 = 2.989 x 10-24 kg
Calculation of Volume at STP
Calculate the volume of following at STP.
8.5 x 10-4 kg of ammonia
Molecular mass of ammonia (NH3) = 14 x 1 + 1 x 3 = 14 + 3 = 17 g
= 17 x 10-3 kg
Number of moles of ammonia = given mass/ molecular mass
= (8.5 x 10-4)/(17 x 10-3) = 0.05
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of ammonia = number of moles x 22.4
Volume of 8.5 x 10-4 kg of ammonia at STP = 0.05 x 22.4 = 1.12 dm3
3.5 x 10-3 kg of nitrogen
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g = 28 x 10-3 kg
Number of moles of nitrogen = given mass/ molecular mass
= (3.5 x 10-3)/(28 x 10-3) = 0.125
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of nitrogen= number of moles x 22.4
Volume of 3.5 x 10-3 kg of nitrogen at STP = 0.125 x 22.4 = 2.8 dm3
14 g of nitrogen
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g = 28 x 10-3 kg
Number of moles of nitrogen = given mass/ molecular mass
= (14 x 10-3)/(28 x 10-3) = 0.5
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of nitrogen= number of moles x 22.4
Volume of 3.5 x 10-3 kg of nitrogen at STP = 0.5 x 22.4 = 11.2 dm3
6.023 x 1022 molecules of ammonia
Number of moles of ammonia = Given molecules/ Avogadro’s number
Number of moles of ammonia = (6.023 x 1022)/(6.023 x 1023) = 0.1
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of ammonia = number of moles x 22.4
Volume of 6.023 x 1022 molecules of ammonia at STP = 0.1 x 22.4 = 2.24 dm3
2.008 x 1023 molecules of SO2 at STP.
Number of moles of SO2 = Given molecules/ Avogadro’s number
Number of moles of SO2 = (2.008 x 1022)/(6.023 x 1023) = 0.3334
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of SO2 = number of moles x 22.4
Volume of 2.008 x 1023 molecules of SO2 at STP = 0.3334 x 22.4 = 7.469 dm3
0.2 mole of sulphur dioxide.
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of sulphur dioxide = number of moles x 22.4
Volume of 0.2 moles of sulphur dioxide at STP = 0.2 x 22.4 = 4.48 dm3
One reply on “Calculation of Mass of a Molecule and an Atom”
It’s really good so please I want more answers .