Science > Chemistry > Chemical Thermodynamics and Energetics > Introduction
Chemical Thermodynamics and its Scope:
Energy stored in chemical substances is called chemical energy. Thermodynamics is the branch of science that deals with the different forms of energy, the quantitative relationships between them and the energy changes that occur in the physical and chemical process. Thermodynamics deals with all types of energies and conversion of one form of energy into other forms. Hence nowadays the term energetics is used in place of thermodynamics. Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics.
Limitations of Thermodynamics:
- Properties such as pressure, temperature, volume, and composition are the properties of matter in bulk. These properties are not based on the arrangement of atoms in molecules and molecular structures. Such properties are called macroscopic properties. Thermodynamics deals with macroscopic properties. It does not make use of any theory about atomic structure and molecular structure. Hence it is called macroscopic science.
- It helps to predict whether the chemical reaction can occur under a given set of conditions but it does not tell anything about the rate of reaction.
- It is more concerned with the initial and final states of the system. It does not tell anything about the mechanism of the process.
- It does not deal with the internal structure of molecules or atoms.
Terminology of Thermodynamics:
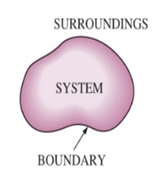
- System: The portion of the physical universe under thermodynamic study is called the system.
- Surroundings: The remaining part of the universe under thermodynamic study is called the surroundings.
- Boundary: The real or imaginary surface separating the system from the surrounding is called the boundary.
The boundary may be real or imaginary. It may be rigid or non-rigid. It may be conducting (diathermic) or non-conducting (adiabatic).
System + Surroundings = Universe
A given amount of one or more substances form the system. Thus, 100 kg of water placed in a flask constitutes the system. The air and flask in contact with water form the surroundings. A system is separated from the surroundings by real or imaginary boundary through which matter and energy can flow from the system to the surroundings and vice-versa.
Diagrammatic representation:
Types of Systems On the Basis of Exchange of Matter and Energy:
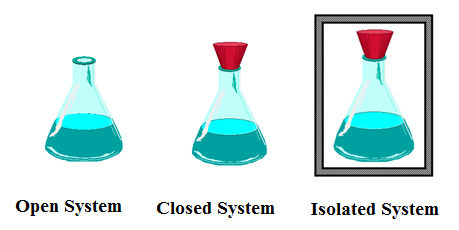
Open System:
A system which can exchange both energy and matter with the surroundings is called an open system.
Example: A hot solution in a beaker is an open system because it can exchange both the matter (vapours) and energy (heat) to the surroundings. All living organisms like plants and vegetables. (Total amount of energy does not remain constant. The mass and the temperature can undergo change).
Characteristics of Open System:
- A system which can exchange both energy and matter with the surroundings is called an open system.
- The total amount of energy does not remain constant.
- The total amount of mass does not remain constant.
Closed system:
A system which can exchange energy but not matter with the surroundings is called a closed system.
Example: Water or gas in a closed (sealed) vessel. The substance can be heated or it can give out heat (energy exchange) but no substance can escape from the vessel. (Total amount of energy does not remain constant. Mass of the system remains constant but the temperature can undergo change)
Characteristics of Closed System:
- A system which can exchange energy but not matter with the surroundings is called a closed system.
- The total amount of energy does not remain constant.
- The total amount of mass remains constant.
Isolated system:
A system which can exchange neither matter nor energy with the surroundings is called an isolated system.
Example: A liquid placed in a thermos flask is an isolated system. Temperature change outside the flask does not change the temperature of the liquid. (no energy transfer) and nothing can escape from or enter the flask (no transfer of matter). (Total amount of energy remains constant. Both the mass and energy of the system constant).
Characteristics of Isolated System:
- A system which can exchange neither energy nor matter with the surroundings is called isolated system.
- The total amount of energy remains constant.
- The total amount of mass remains constant e.g. hot solution kept in
Types of Systems On the Basis of Phases of Matter (composition):
Homogeneous system:
When a system is a uniform throughout or consists of a single-phase, it is said to be the homogeneous system.
Examples: A pure single solid, liquid or a gas. A mixture of gases. The true solution of a solid in the liquid.
Characteristics of Homogeneous System:
- When a system is a uniform throughout or consists of a single-phase, it is said to be the homogeneous system.
- This system contains only a single phase.
- This system is uniform throughout and hence there is no separation boundary between the constituents of the system
Heterogeneous system:
A heterogeneous system is one which is not uniform throughout and contains two or more phases which are separated from one another by definite boundary surface.
Examples: Mixture of two immiscible liquids such as benzene and water, The mixture of two or more solids.
Characteristics of Heterogeneous System:
- When a system is not uniform and contains three or more phases, it is said to be a heterogeneous system.
- This system contains two or more phases. The phases in this system are separated from one another by a definite boundary surface.
- The phases in this system are separated from one another by a definite boundary surface.
Universe as Isolated system:
The universe can be considered as an isolated system due to the following reasons
- The total mass and energy of the universe are conserved.
- The universe has no boundary, hence it has no surroundings.
- There is an interchange between different forms of energy due to natural and arranged processes within the universe.
- The natural or arranged process may be exothermic or endothermic due to which there is a change of temperature as it takes place in an isolated system.
Properties of System:
Extensive Properties:
A property that depends on the amount (or amounts) of the substance (or substances) present in the system is called extensive properties.
Examples: Volume, Mass and Energy are extensive properties.
Intensive Properties:
An intensive property of a system is one which is independent of the amount of the system and is a specific characteristic of the system.
Examples: Refractive index, density, surface tension.
State of a System:
To describe the system completely and ambiguously, macroscopic properties such as pressure, volume, temperature, mass (number of moles) and composition are used. By assigning numerical values to these properties, the state of a system can be defined.
State Function:
Any property of a system whose value depends on the current state of the system and is independent of the path followed to reach that state is called the state function.
Examples: Pressure (P), volume (V), Temperature (T) Internal energy (E) Enthalpy (H).
Characteristics of State Functions:
- If the values of some state function variables are changed, values of all other variables get adjusted automatically.
- When the state of the system is altered, the values of state functions change. the change in a state function (say x) ΔX, depends only on the state of a system before alteration (initial state) and that after alteration (final state) and is given by
Δ X = Xfinal – Xinitial = X2 – X1
- ΔX is independent of the manner ( i.e. path) in which the state is altered
- ΔX is independent of the manner ( i.e. path) in which the state is altered
Significance of State Functions:
State functions are important thermodynamic property because it depends on initial and final states of the system but independent of the path followed by the system to bring about the change. The feasibility of a process can be verified using state functions because they are independent of the path followed by the system to bring about the change. For example, when ΔG is negative the process is spontaneous and can take place on its own when initiated. While when ΔG is positive the process is non-spontaneous and is to be arranged externally.
Path Function:
The property of the system whose value depends on the path used to reach a particular state is called a path function.
Examples: Work (W), Heat (q)
Thermodynamic Equilibrium:
A system is said to be in a state of thermodynamic equilibrium when the state functions of the system do not change with time. Thermodynamics deals with the system which is in states of thermodynamic equilibrium. For such a state, the following three equilibria should exist simultaneously in the systems.
Thermal equilibrium: No change of temperature with time.
Chemical equilibrium: No change in chemical composition with time.
Mechanical equilibrium: No macroscopic movement i.e. no unbalanced force should act on or from within the system. Pressure remains constant though out in all parts of the system.
Different Types of Thermodynamic Equilibrium:
In chemical thermodynamics, for thermodynamic equilibrium, the system has to attain the following three types of equilibrium.
Thermal Equilibrium:
A system is said to be in thermal equilibrium with the surroundings when the system and surroundings are at the same temperature and there is no exchange of heat energy between them. In such an equilibrium total, the internal energy of the system remains constant.
Example: water in equilibrium with its vapours at a constant temperature.
Chemical Equilibrium:
A system is said to be in chemical equilibrium when the chemical composition of reactants and products do not change with time. Thus the chemical composition of a system as a whole remains constant. In such an equilibrium, the reaction does not stop it continues but the rate of the forward reaction is equal to the rate of backward reaction.
Example: N2(g) + 3 H2(g) ⇌ 2NH3(g)
Mechanical Equilibrium:
A system is said to be in mechanical equilibrium when net force acting on the system is zero and the net moment of the system is zero. In such equilibrium, the system neither has translational motion nor has rotational motion.
Example: Column of a structure
Next Topic: Types of Chemical Processes
7 replies on “Introduction to Chemical Thermodynamics”
Thank u sir good websites
It’s great
Sir, Basically Im a commerce graduate, During this lockdown, I chose this site to teach about thermodynamics to my son who is in +1, I could understand the concept as its been in a Very clear and detailed explanation, Thank you so much.
Good and understandable notes,, good work
very impressive
What a didactic and detailed presentation. I am quite impressed. Thank you sir.
Thanks indeed, I found these material so vital to my cause.