Science > Chemistry > Chemical Thermodynamics and Energetics > Concept of Entropy of a System
In this article, we shall study the concept of entropy and its relation with the spontaneity.
Spontaneous Process:
The spontaneous process is defined as a process that takes place on its own without external influence. A spontaneous process does not mean a fast process. For example, take the case of a combination of hydrogen and oxygen. These gases may be mixed at room temperature and left for many years without observing any perceptible change. Although the reaction is taking place between them, it is at an extremely slow rate. It is still called a spontaneous reaction. So spontaneity means having the potential to proceed without the assistance of an external agency.
Spontaneity does not tell about the rate of the reaction or process. Another aspect of spontaneous reaction or process, as we see is that these cannot reverse their direction on their own. We may summarize it as follows: A spontaneous process is an irreversible process and may only be reversed by some external agency. All spontaneous process takes place in the direction to decrease in energy and to attain equilibrium.
Examples of Spontaneous Processes:
- Water always flows from a higher level to a lower level on its own but cannot flow from lower level to higher level on its own.
- Heat always flows from the body at a higher temperature to body at lower temperature on its own it cannot flow from the body at a lower temperature to body at a higher temperature on its own
Characteristics of Spontaneous Process:
- Once the spontaneous process starts, it proceeds without continuous external help.
- For spontaneity of reaction, the products must be more stable than the reactants.
- For spontaneity of reaction, the product should have less energy than the reactants.
- All spontaneous process takes place in the direction to decrease in energy and to attain equilibrium.
- Spontaneous process increase disorder in the system thereby increasing the entropy of the system.
- the spontaneous process cannot reverse their direction on their own. Thus a spontaneous process is an irreversible process and may only be reversed by some external agency.
- The spontaneous process proceeds until an equilibrium is reached.
- Generally, spontaneous processes are exothermic but there are some processes which are spontaneous but endothermic. e.g. melting of ice.
- The rate of a spontaneous process may be fast or slow.
Non-spontaneous Process:
A non-spontaneous process is defined as a process which does not take place on its own but can take place under the external influence.
Characteristics of Non-Spontaneous Process:
- Non-spontaneous processes are not natural.
- They can not takes place on their own.
- An external agency is required to carry out such reactions.
- These processes are generally accompanied by the absorption of energy. i.e. generally they are endothermic
- In the non-spontaneous process, there is an increase in enthalpy, a decrease in entropy and an increase in Gibb’s energy.
Energy and Spontaneity:
The substances having more energy are less stable than the substances having low energy. In a spontaneous reaction, the substances with more energy are converted into substances of low energy. In general, all spontaneous process takes place in the direction to decrease in energy and to attain equilibrium. Thus reaction to be spontaneous, it should be exothermic.
Example: Acid-base neutralization is spontaneous because it is exothermic. Exothermic nature assists spontaneity but is not the sure criteria of spontaneity. Melting of ice and the formation of a solution of NaCl in water are endothermic processes but are spontaneous.
Concept of Entropy:
Entropy is a thermodynamic property which determines spontaneity of the process. Consider the melting of ice. In this process, the ordered solid state gets converted into a disordered liquid state. Similarly, during the vaporization of water, the ordered liquid state gets converted into the disordered gaseous state. Thus in both the cases discussed above, there is an increase in molecular disorder. This randomness of molecules is measured by a thermodynamic property called entropy.
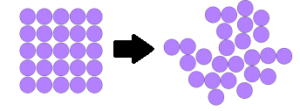
When there is an increase in disorderedness then change in ΔS is positive. ΔS > 0. When there is a decrease in disorderedness then change in ΔS is negative. ΔS < 0.
The absolute value of entropy cannot be calculated. The entropy change of a system in a process is equal to the amount of heat transferred to it in a reversible manner divided by the temperature at which the transfer takes place. When heat is supplied to a system the disorder increases. Thus the change in entropy ΔS is directly proportional to heat supplied. It is also observed that the change in entropy ΔS is inversely proportional to the temperature at which the heat addition takes place.
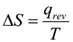
Entropy is a state function. The unit of ΔS is J K-1mol-1.
Entropy and Spontaneity:
In most of the cases, the entropy of a system increases in a spontaneous process. But there are some spontaneous processes in which it decreases. This discrepancy is explained in the second law of thermodynamics which states that “the total entropy of the system and its surroundings (universe) increase in a spontaneous process
Thus, SUniverse = STotal = ΔSSystm + ΔSSurroundings
For spontaneous process ΔSTotal > 0.
The spontaneity of reaction can be determined by the following relations.
- If ΔSTotal > 0, the process is spontaneous
- If ΔSTotal < 0, the process is non-spontaneous
- If ΔSTotal = 0, the process is in equilibriums
Entropies of Phase Transformation:
Entropy of Fusion:
It is defined as the entropy change taking place when one mole of a substance changes from a solid state into a liquid state at its melting point.
Solid ⇌ Liquid
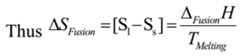
Since ΔSFusion is positive. Hence entropy of liquid state is greater than that of solid state.
Entropy of Vapourization:
It is defined as the entropy change taking place when one mole of a substance changes from a liquid state into a gaseous state at its boiling point.
Liquid ⇌ Gas

Since ΔSvap is positive. Hence entropy of gaseous state is greater than that of the liquid state.
Previous Topic: Hess’s Law and its Applications
Next Topic: Concept of Gibb’s Free Energy