Science > Chemistry > Concept of Atomic Mass and Equivalent Mass > Use of Laws of Electrolysis
In the last few articles, we have studied the hydrogen displacement method, oxide formation method, reduction method, chloride formation method, and double displacement method to determine the equivalent mass of metal. In this article, we shall study use of Faraday’s laws of electrolysis to determine the equivalent mass of a substance.
The equivalent mass of a substance is the number of parts by mass of the substance which combines with or displaces or contains 1.008 parts by mass of hydrogen, 8 part by mass of oxygen, or 35.5 part by mass of chlorine. If the equivalent mass is expressed in grams then it is called gram equivalent mass (GEM).
Illustration:
1.008 parts by weight of Hydrogen combines with 35.5 parts by weight of chlorine to give 36.5 parts by weight of HCI. Thus the equivalent mass of chlorine is 35.5.
Equivalent mass has no unit because it is a pure ratio. Some important equivalent masses are H = 1, O = 8, Cl = 35.5
Method – VII (Faraday’s First Law of Electrolysis):
Statement :
The mass of any substance deposited or liberated or dissolved at an electrode during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte.
Steps Involved:
A known quantity of electricity (Q = i t) is passed through electrolyte solution and mass (w) of the substance deposited or liberated during electrolysis is measured.
Using the following relation value of electrochemical equivalent (z) is calculated.
w = z i t
Then equivalent mass is calculated by the formula
Equivalent mass = 96500 x z
Numerical Problems on faraday’s First Law of Electrolysis
Example – 01:
On passing a current of 0.5 A through a solution of a salt of a metal for 32 minutes 0.3158 g of the metal was deposited. What is the equivalent mass of the metal?
Given: i = 0.5 A, t = 32 min = 32 x 60 s , W = 0.3158
Solution:
By Faraday’s first law of electrolysis,
W = z i t
z = W/ (i t) = 0.3158/(0.5 x 32 x 60)
z = 3.29 x 10-4 g/C
Now, Equivqlent mass = 96500 x z
Equivalent mass = 96500 x 3.29 x 10-4 = 31.74
Ans: The equivalent mass of metal is 31.74
Example – 02:
On passing a current of 0.6 A through a solution of a salt of copper for 20 minutes 0.24 g of the copper was deposited. What is the equivalent mass of copper?
Given: i = 0.6 A, t = 20 min = 20 x 60s, W = 0.24 g
Solution:
By Faraday’s first law of electrolysis,
W = z i t
z = W/ (i t) = 0.24/(0.6 x 20 x 60)
z = 3.33 x 10-4 g/C
Now, Equivalent mass = 96500 x z
Equivalent mass = 96500 x 3.33 x 10-4 = 31.84
Ans: The equivalent mass of copper is 31.84
Method – VIII (Faraday’s Second Law of Electrolysis):
Statement:
When the same quantity of electricity is passed through different electrolytes (generally connected in series), the masses of different substances deposited or liberated or dissolved at the respective electrodes are directly proportional to their chemical equivalents (equivalent masses).
Steps Involved:
The same quantity of electricity is passed through the solution of different electrolytes, the masses of different substances liberated or evolved as a result of electrolysis are noted.
Then equivalent mass is calculated by the formula.
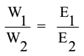
Where W1 = mass of the first substance deposited
W2 = mass of the second substance deposited
E1 = Equivalent mass of the first substance
E2 = Equivalent mass of the second substance
Numerical Problems on faraday’s Second Law of Electrolysis
Example – 01:
An electric current is passed through two cells containing CuSO4 and AgNO3 solutions respectively connected in series. The masses of copper and silver deposited are 0.424 g and 1.44 g respectively. Find the equivalent mass of copper if that of silver is 108.
Given: WCu = 0.424 g, WAg = 1.44 g, EAg = 108
To Find: ECu =?
Solution:
By Faraday’s second law of electrolysis,

Ans: The equivalent mass of copper is 31.8
Example – 02:
The same quantity of electricity that liberated 2.158 g of silver was passed through a solution of a gold salt and 1.314 g of gold was deposited. The equivalent mass of silver is 107.9. Calculate the equivalent mass of gold. also find oxidation state and valency of gold.
Given: WAg = 2.158 g, WAu = 1.314 g, EAg = 107.9
To Find: EAu =?
Solution:
By Faraday’s second law of electrolysis,
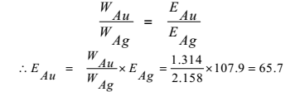

Ans: The equivalent mass of gold is 65.7 and its oxidation state is+3. Valency is 3.