Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Factors Governing Formation of Ionic Bond
In this article, we shall study the factors affecting the formation of the ionic bond and the concept of variable electrovalency.
Ionization energy of Electropositive Atom:
It is defined as the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom of an element. The ionization energy of an atom can be considered as a measure to lose an electron and form a cation. The lesser the ionization energy, the greater is the ease of the formation of a cation and thus it can form ionic bond easily.
Alkali metals and alkaline earth metals have low ionization energy hence they have a tendency to form ionic compounds.
Electron Affinity or Electron Gain Enthalpy of Electronegative Atom:
It is defined as the amount of energy released when an electron is added to an isolated gaseous atom of an element. The electron gain enthalpy of an atom can be considered as a measure to gain an electron and form an anion. The higher the energy released during this process, the easier will be the formation of an anion.
Elements of groups 16 and 17 have more negative values of electron gain enthalpies hence they have a tendency to form ionic compounds.
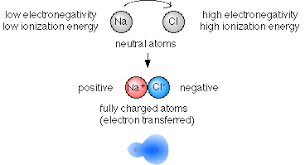
Thus, the low ionization energy of a metal atom and high electron affinity of a non-metal atom facilitate the formation of an ionic bond between them.
Lattice energy or Lattice Enthalpy:
The enthalpy change involved in the formation of one mole of an ionic crystal from its constituent gaseous positive and negative ion is called lattice energy. The formation of an ionic compound is an exothermic process. Hence the enthalpy change involved in the process of formation of an ionic compound is negative.
Higher the lattice energy, the greater is the tendency of the formation of an ionic bond. The higher the charges on the ions and the smaller the distance between them, the greater is the force of attraction between them.
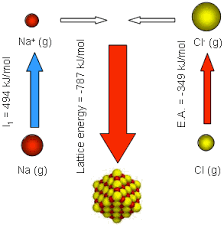
The lattice energy depends on the following factors
- Size of the atom: If the ions formed are smaller in size, the inter-nuclear distance is less and hence the inter-ionic attractive force is greater. Hence the lattice energy will be high. Thus smaller the size of ions, greater is the lattice energy.
- Charge on the ions: Higher the charges on the ion, greater the inter-ionic attractive force. Hence lattice energy is high. Thus higher the charge on the ions, greater is the lattice energy.
Difference in Electronegativities:
The tendency of an atom to attract the bonding or a shared pair of electrons towards its own side in a covalent bond is called electronegativity of that atom. Higher the difference in electronegativities of the two atoms, greater will be the ease to form an ionic bond.
A difference of 2 units (pauling) in electronegativities is required to form an ionic bond.
The Concept of Variable Electrovalency:
There are many elements in the periodic table which are capable of forming more than one type of ions having different charges. Thus they possess more than one electrovalency. This phenomenon is known as a variable electrovalency. For example, copper forms cuprous (Cu+) and cupric (Cu 2+) ions. Iron forms ferrous (Fe2+) and ferric (Fe3+) ions.
Causes of Variable Electrovalency:
Unstable Nature of Core (Kernel):
When an atom loses one or more electrons, a cation is formed. The remaining part of the atom left is called a core or a kernel of the atom.
During the formation of positive ion-neutral atom loses one or more (definite) number of electrons to form a cation. If core or kernel form is stable then it will exhibit definite electrovalency.
Na → Na+ + e–
1s2 2s2 2p6 3s1 1s2 2s2 2p6
Sodium atom core or kernel of sodium
If core or kernel of ion formed is not stable then to acquire greater stability it is compelled to lose more electrons. This fact gives rise to variable electrovalency. Let us consider the case of iron Fe (Z = 26)
Fe → Fe 2+ + 2 e–
1s2 2s2 2p6 3s2 3p6 3d6 4s2 1s2 2s2 2p6 3s2 3p6 3d6
(2, 8, 14, 2) (2, 8, 14) less stable
Fe2+ → Fe 3+ + e–
1s2 2s2 2p6 3s2 3p6 3d6 1s2 2s2 2p6 3s2 3p6 3d5
(2, 8, 14) (2, 8, 13)
more stable due to exactly half filled d orbitals
Inert Electron Pair Effect:
The reluctance of ns2 electron pair to get excited and to take part in bond formation is called inert electron pair effect.
Let us consider case of tin Sn (Z = 50). Electronic configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. (2, 8, 18, 18, 4). It has 4 electrons and can form Sn4+ ion by donating 4 electrons and acquires configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 (2, 8, 18, 18).
But due to reluctance of 5s2 electron pair to get excited and to take part in bond formation, it loses only two electrons and acquires configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 (2, 8, 18, 2) an forms Sn2+ ion.
Thus tin shows two electrovalencies due to the inert pair effect.