Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Properties of Ionic Compounds
In this article, we shall study the properties of ionic compounds.
Physical State:
Due to strong electrostatic force present between the oppositely charged ions, they are held closer and fixed at specified positions in the crystal lattice. Hence ionic compounds usually exist in the form of crystalline solids at room temperature. Actually, these compounds do not possess a molecule but it is a well-defined geometric arrangement of positive and negative ions in the crystal lattice.
In sodium chloride (NaCl), each Na+ ion is surrounded by 6 Cl– ions and each Cl– ion is surrounded by 6 Na+ ions. The arrangement is as shown below.
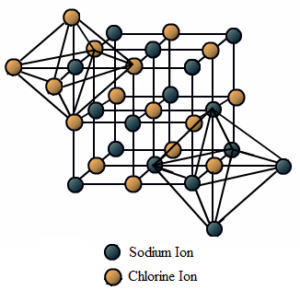
High Melting and Boiling Points of Ionic Compounds:
Due to strong electrostatic force present between the oppositely charged ions, they are held closer and fixed at specified positions in the crystal lattice. Hence very high temperature is required to separate from each other and to move freely as in the case of liquid. Hence the boiling and melting points of ionic compounds are generally very high.
Hard and Brittle Nature of Ionic Compounds:
Due to strong electrostatic force present between the oppositely charged ions, they are held closer and fixed at specified positions in the crystal lattice. There are layers of positive ions and negative ions. When force is applied to them the layer may slide and the ions having similar charges may come near each other. In such a case, the two layers repel each other and the crystal gets cleaved. Hence ionic solids are brittle.
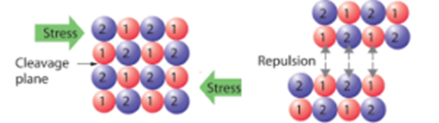
Electrical Conductivity:
Due to strong electrostatic force present between the oppositely charged ions, they are held closer and fixed at specified positions in the crystal lattice. Hence in solid-state ionic compounds do not conduct electricity. In the Fused state or the dissolved state, the ions of the ionic compound can move from one position to another freely. Hence in fused state, ionic compounds conduct electricity.
Lattice Energy (ΔlatticeH):
The enthalpy change involved in the formation of one mole of an ionic crystal from its constituent gaseous positive and negative ion is called lattice energy. The formation of an ionic compound is an exothermic process. Hence the enthalpy change involved in the process of formation of an ionic compound is negative. The lattice energy decreases with the increase in the ionic size.
Hydration Energy (ΔhydrationH|):
The enthalpy change involved in the hydration of one mole of gaseous ions of each type of an ionic compound (solid) is called hydration energy. Hydration of ionic compounds is exothermic. Hence the enthalpy change involved in the process of hydration of an ionic compound is negative. The hydration energy decreases with the increase in the ionic size.
Solubility in water:
Water has a high dielectric constant hence it weakens the electrostatic force between the ions of an ionic compound. Due to which the ions get separated and get surrounded by the water molecule. This dissolves the ionic compound in water. Hence ionic compounds are soluble in water.
Organic solvents like benzene, ether, hexane have low dielectric constant and are unable to weaken the electrostatic force between the ions of the ionic compound. Thus they are unable to separate the ions. Hence ionic compounds are not soluble in organic solvents.
The enthalpy of solution of an ionic solid is numerically equal to the difference in its hydration energy and lattice energy
Mathematically, ΔsolutionH = ΔhydrationH – ΔlatticeH
The Gibb’s energy (free energy) change is given by
ΔG = ΔH – T ΔS i.e.
ΔsolutionG = ΔsolutionH – T ΔsolutionS
Hence for a compound to get dissolved in water, Gibb’s energy (free energy) change involved in the solution formation process is negative. Thus the quantity (ΔsolutionH – T ΔsolutionS) is negative. The quantity ΔsolutionS is positive i.e. the quantity T ΔsolutionS is positive. It means the quantity ΔsolutionH should be smaller than T ΔsolutionS. Hence a more negative value of ΔsolutionH will help the greater dissolution of an ionic compound in water.
Hence we can conclude that
- If ΔhydrationH > ΔlatticeH, the salt would dissolve in water.
- If ΔhydrationH < ΔlatticeH, ordinarily the salt would dissolve in water. It will start dissolving when ΔsolutionH < T ΔsolutionS
Stereoisomerism:
Isomers having the same molecular formula, same structural formula but different configurations are called stereo Isomers and the phenomenon is known as stereoisomerism. The ionic compounds are neither rigid or directional hence they do not show stereoisomerism.
Ionic Reactions:
The ionic reaction involves the mutual interaction of ions. Due to the presence of ions, the reactions of ionic compounds are very fast
Born-Haber Cycle
The energy change involved in the formation of ionic bonds from the constituent elements can be represented by a cycle called Born-Haber Cycle. It uses Hess’s law of thermodynamics to calculate the change in enthalpy during ionic bond formation.
Let us consider the formation of 1 mole of sodium chloride from sodium and chlorine.

The Born-Haber Cycle can be simply stated as Heat of formation of ionic crystal = Heat of atomization + Dissociation energy+ (sum of Ionization energies)+ (sum of Electron affinities) + Lattice energy.
If energy is released (exothermic reaction), put a negative sign in front of the value of the energy; if energy is absorbed (endothermic reaction), the value of energy should be positive.
Note:
In this general equation, the electron affinity is added. However, when plugging in a value, determine whether energy is released (exothermic reaction) or absorbed (endothermic reaction) for each electron affinity.