Science > Chemistry > Chemical Thermodynamics and Energetics > Hess’s Law and its Applications
In this article, we shall study Hess’s law and its applications in thermochemistry.
Statement :
It states that the change in enthalpy accompanying a chemical reaction is independent of the pathway between initial and final states of the chemical reaction.
Explanation:
Let us suppose substance ‘A’ is converted into D directly.
A → D, ΔH = Q kJ
Suppose the same change is brought about in different steps,
i) A → B, ΔH1 = Q1 kJ
ii) B → C, ΔH2 = Q2 kJ
iii) C →D , ΔH3 = Q3 kJ
Representation:
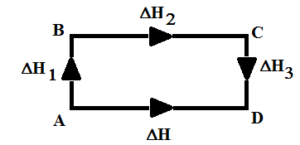
Then, according to Hess’s law,
ΔH = ΔH1 + ΔH2 + ΔH3
Q = Q1 + Q2 + Q3
Thus, Hess’s law implies that change in enthalpy of a chemical reaction depends upon the initial and final state of a chemical reaction irrespective of the number of steps involved in a chemical reaction.
Illustration:
The change in enthalpy of formation of carbon dioxide can be determined in two ways.
One-step preparation:
Solid carbon is burnt in excess of oxygen.
C(s) + O2(g) → CO2(g) , Δ H = -395.39 kJ
Preparation in two steps:
Step – 1: Solid carbon is burnt in limited supply of oxygen to form carbon monoxide,
C(s) + 1/2O2(g) → CO(g) , Δ H1 = -111.7 kJ
Step – 2: Carbon monoxide is then burnt in excess of oxygen to from carbon dioxide,
CO(g)+ 1/2O2(g) → CO2(g , ΔH2= -283.67 kJ
According to Hess’s law,
Δ H1 + ΔH2 = -111.7 kJ – 283.67 kJ = -395.39 kJ = ΔH
Thus Hess’s law is illustrated.
Applications of Hess’s Law:
- Thermochemical equations can be added subtracted or multiplied like ordinary algebraic equations.
- Hess’s law is useful to calculate heats of many reactions which do not take place directly.
- It is useful to find out heats of extremely slow reaction.
- It is useful to find out the heat of formation, neutralization, etc.
Numerical Problems Based on Hess’s Law:
Example – 01:
Calculate the enthalpy of formation of CO from given data
i) C(s) + O2(g) → CO2(g) ΔH° = -393.5 KJ
ii) CO(g)+ ½O2(g) → CO2(g) ΔH° = – 283.0 KJ
Solution:
The formation of carbon monoxide is represented by following thermochemical equation
C(s) + ½O2(g) → CO(g) ΔH° = ?
Keeping given equation (i) as it is and reversing equation (ii) we get
i) C(s) + O2(g) → CO2(g) ΔH1° = -393.5 KJ
iii) CO2(g) → CO(g)+ ½O2(g) ΔH2° = + 283.0 KJ
Adding equations (i) and (iii) and by Hess’s Law we get
C(s) + ½O2(g) → CO(g) ΔH° = ΔH1° + ΔH2° = -393.5 KJ + 283.0 KJ
C(s) + ½O2(g) → CO(g) ΔH° = -110.5 KJ
Ans: Hence enthalpy of formation of CO is – 110.5 kJ mol-1
Example – 02:
Calculate the enthalpy of formation of CH4 from given data
i) C(s) + O2(g) → CO2(g) ΔH° = -393.5 KJ
ii) H2(g) + ½O2(g) → H2O(l) ΔH° = – 285.8 KJ
iii) CH4(g) + 2O2(g) → CO2(g) + 2 H2O(l) ΔH° = – 890.3 KJ
Solution:
The formation of CH4 is represented by following thermochemical equation
C(s) + 2H2(g) → CH4(g) ΔH° = ?
Keeping given equation (i) as it is, multiplying equation (ii) by 2 and reversing equation (ii) we get
i) C(s) + O2(g) → CO2(g) ΔH1° = -393.5 KJ
iv) 2 H2(g) + O2(g) → 2 H2O(l) ΔH2° = – 571.6 KJ
v) CO2(g) + 2 H2O(l) → CH4(g)+ 2O2(g) ΔH3° = + 890.3 KJ
Adding equations (i) (iv) and (v) and by Hess’s Law we get
C(s) + 2H2(g) → CH4(g) ΔH° = ΔH1° + ΔH2° + ΔH3° = -393.5 KJ – 571.6 KJ + 890.3 kJ
C(s) + 2H2(g) → CH4(g) ΔH° = – 74.8 KJ
Ans: Hence enthalpy of formation of CH4 is – 74.8 kJ mol-1
Example – 03:
Calculate the ΔH° for the reaction
2 ClF(g) + O2(g) → Cl2O(g) + OF2(g)
From following equations
i) F2(g) + ClF(g) → ClF3(l) ΔH° = – 139.2 KJ
ii) 2 ClF3(l) + 2 O2(g) → Cl2O(g) + 3 OF2(g) ΔH° = + 533.4 KJ
iii) F2(g) +½O2(g) → OF2(g) ΔH° = + 24. 7 KJ
Solution:
Required reaction is
2 ClF(g)+ O2(g) → Cl2O(g) + OF2(g)
Multiplying equation (i) by 2, keeping equation (ii) as it is, and multiplying equation (iii) by 2 and reversing it.
iv) 2 F2(g) + 2 ClF(g) → 2 ClF3(l) ΔH° = – 278.4 KJ
ii) 2 ClF3(l) + 2 O2(g) → Cl2O(g) + 3 OF2(g) ΔH° = + 533.4 KJ
v) 2 OF2(g) → 2 F2(g)+ O2(g) ΔH° = – 49.4 KJ
Adding equations (iv) (ii) and (v) and by Hess’s Law we get
2 ClF(g)+ O2(g) → Cl2O(g) + OF2(g) ΔH° = ΔH1° + ΔH2° + ΔH3° = – 278.4 KJ + 533.4 KJ – 49.4 kJ
2 ClF(g) + O2(g) → Cl2O(g) + OF2(g) ΔH° = + 205.6 KJ
Hence ΔH° for the reaction is 205.6 kJ
Example – 04:
Calculate the ΔH° for the reaction between ethene with water to form ethanol from the following data.
From following equations
i) C2H5OH(l) + 3 O2(g) → 2CO2(g) + 3 H2O(l) ΔH° = – 1368 KJ
ii) C2H4(g) + 3 O2(g) → 2CO2(g) + 2 H2O(l) ΔH° = – 1410 KJ
Is ΔH° calculated the enthalpy of formation of liquid ethanol?
Solution:
The reaction between ethene and water is represented by
C2H4(g) + H2O(l) → C2H5OH(l) ΔH° = ?
Reversing equation (i), keeping equation (ii) as it is
iii) 2CO2(g) + 3 H2O(l) → C2H5OH(l) + 3 O2(g) ΔH° = + 1368 KJ
ii) C2H4(g) + 3 O2(g) → 2CO2(g) + 2 H2O(l) ΔH° = – 1410 KJ
Adding equations (iii) and (ii) and by Hess’s Law we get
C2H4(g) + H2O(l) → C2H5OH(l) ΔH° = ΔH1° + ΔH2° = 1368 KJ -1410 KJ
C2H4(g) + H2O(l) → C2H5OH(l) ΔH° = – 42 kJ
Hence ΔH° for the reaction is – 42 kJ
This cannot be the heat of formation of ethanol, because it is not obtained from its constituent elements in their standard state.
Example – 05:
Calculate the ΔH° for the reaction 2C(graphite) + 3 H2(g) → C2H6(g)
From following equations
i) C2H6(g) + 7/2 O2(g) → 2CO2(g) +3 H2O(l) ΔH° = – 1560 KJ
ii) H2(g) + 1/2 O2(g) → H2O(l) ΔH° = – 285.8 KJ
ii) C(graphite) + O2(g) → CO2(g) ΔH° = – 393.5 KJ
Solution:
The required reaction is
2C(graphite) + 3 H2(g) → C2H6(g) ΔH° =?
Reversing equation (i), multiplying equation (ii) by 3 and multiplying equation (iii) by 2 we get
iv) 2CO2(g) +3 H2O(l) → C2H6(g) + 7/2 O2(g) ΔH° = + 1560 KJ
v) 3H2(g) + 3/2 O2(g) → 3 H2O(l) ΔH° = – 857.4 KJ
vi) 2C(graphite) + 2O2(g) → 2CO2(g) ΔH° = – 787.0 KJ
Adding equations (iv), (v) and (vi) and by Hess’s Law we get
2C(graphite) + 3 H2(g) → C2H6(g) , ΔH° = ΔH1° + ΔH2° + ΔH3° = 1560 KJ -857.4 KJ – 787 kJ
2C(graphite) + 3 H2(g) → C2H6(g) ΔH° = – 84.4 kJ
Ans: Hence ΔH° for the reaction is – 84.4 kJ
Example – 06:
Calculate the standard enthalpy for the reaction
2Fe(s) + 3/2 O2(g) → Fe2O3(s)
From following equations
i) 2 Al(s) + Fe2O3(s) → 2Fe(s) + Al2O3(s) ΔH° = – 847.6 KJ
ii) 2 Al(s) + 3 /2 O2(g) → Al2O3(s) ΔH° = – 1670 KJ
Solution:
The required reaction is
2Fe(s) + 3/2 O2(g) → Fe2O3(s) ΔH° =?
Reversing equation (i), keeping equation (ii) as it is we get
iii) 2Fe(s) + Al2O3(s) → 2 Al(s) + Fe2O3(s) ΔH° = 847.6 KJ
ii) 2 Al(s) + 3 /2 O2(g) → Al2O3(s) ΔH° = – 1670 KJ
Adding equations (iii) and (ii) we get
2Fe(s) + 3/2 O2(g) → Fe2O3(s) ΔH° = ΔH1° + ΔH2° = 847.6 KJ – 1670 KJ
2Fe(s) + 3/2 O2(g) → Fe2O3(s) , ΔH° = – 822.4 kJ
Hence standard enthalpy of the reaction is – 822.4 kJ
Example – 07:
Given the following equations and ΔH° at 25 °C
i) Si(s) + O2(g) → SiO2(s) ΔH° = – 911 KJ
ii) 2C(graphite) + O2(g) → 2CO(g) ΔH° = – 221 KJ
iii) Si(s) + C(graphite) → SiC(s) ΔH° = – 65.3 KJ
Calculate ΔH° for the reaction SiO2(s) + 3 C(graphite) → SiC(s) + 2CO(g)
Solution:
The required reaction is
SiO2(s) + 3 C(graphite) → SiC(s) + 2CO(g) ΔH° =?
Reversing equation (i), keeping equations (ii) and (iii) as it is we get
iv) SiO2(s) → Si(s) + O2(g) ΔH° = 911 KJ
ii) 2C(graphite) + O2(g) → 2CO(g) ΔH° = – 221 KJ
iii) Si(s) + C(graphite) → SiC(s) ΔH° = – 65.3 KJ
Adding equations (iv), (ii) and (iii) and by Hess’s Law we get
SiO2(s) + 3 C(graphite) → SiC(s) + 2CO(g) ΔH° = ΔH1° + ΔH2° + ΔH3° = 911 kJ -221 kJ -65.3 kJ
SiO2(s) + 3 C(graphite) → SiC(s) + 2CO(g) ΔH° = + 624.7 kJ
Hence ΔH° for the reaction is 624.7 kJ
Example – 08:
Given the following equations and ΔH° at 25 °C
i) 2H3BO3(aq) → B2O3(s) + 3 H2O(l) ΔH° = + 14.4 KJ
ii) H3BO3(aq) → HBO2(aq) + H2O(l) ΔH° = – 0.02 KJ
iii) H2B4O7(s) → 2 B2O3(s) + H2O(l) ΔH° = 17.3 KJ
Calculate ΔH° for the reaction H2B4O7(s) + H2O(l) → 4 HBO2(aq)
Solution:
The required reaction is
H2B4O7(s) + H2O(l) → 4 HBO2(aq) ΔH° =?
Reversing equation (i) and multiplying by 2, Multiplying equations (i) by4 keeping equation (iii) as it is we get
iv) 2B2O3(s) + 6 H2O(l) → 4 H3BO3(aq) ΔH° = – 28.8 KJ
v) 4 H3BO3(aq) → 4 HBO2(aq) + 4 H2O(l) ΔH° = – 0.08 KJ
iii) H2B4O7(s) → 2 B2O3(s) + H2O(l) ΔH° = 17.3 KJ
Adding equations (iv), (v) and (iii) and by Hess’s Law we get
H2B4O7(s) + H2O(l) → 4 HBO2(aq) ΔH° = ΔH1° + ΔH2° + ΔH3° = – 28.8 kJ – 0.08 kJ + 17.3kJ
H2B4O7(s) + H2O(l) → 4 HBO2(aq) ΔH° = – 11.58 kJ
Hence ΔH° for the reaction is -11.58 kJ
Previous Topic: Concept of Bond Enthalpy
Next Topic: Concept of Entropy of a System
6 replies on “Hess’s Law and its Applications”
It’s very useful which placed above was written in simple english. Thanks.
The examples of Hess’ law application given above was really helpful. Thank you
You are so amazing you make me love chemistry and easier my studies
It is really helpful
Thanks for your consideration
Thank you