Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Octet Theory
The force of attraction which keeps atoms or ions together in a molecule is called a Chemical bond. The main cause of chemical combination is the tendency to acquire stability i.e. a state with minimum energy and a tendency to acquire noble gas configuration in outermost orbit. Many theories are given to explain the nature and types of chemical bonds. In this article, we shall study the octet theory and different types of bonds based on it.
Types of Chemical Bond:
- Electrovalent or ionic bond
- Covalent bond
- Coordinate or Dative bond
- Metallic bond
Octet theory or Octet Rule:
It was put forward by Kossel and Lewis (1916). It states that
- Electronic configuration of noble gases is most stable. Except for helium, all other noble gases have eight electrons in the outermost shell.
- Atoms combine with each other by loss, gain or sharing of electrons so as to acquire an octet of electrons (i.e. 8 electrons) in their outermost shell to attain configuration of nearest noble (inert)
Lewis Structure:
Electrons in inner orbit do not take part in a chemical reaction. For forming bonds only electrons in outermost orbit are involved. The electrons present in the outermost shell of an atom are called valence electrons. Lewis introduces a simple notation to represent these valence electrons by dots. Only valence electrons are shown as dots surrounding the symbol of the given element.
Classification of Chemical Bonds on the Basis of Octet Theory:
According to octet theory, chemical bonds are classified into three types as :
- Ionic or Electrovalent bond.
- Covalent bond.
- Coordinate bond.
Ionic or Electrovalent Bond:
The electrostatic force of attraction between the oppositely charged ions is called the ionic bond or electrovalent bond. In this type of bond formation, there is a transfer of electrons from the outermost shell of one atom (generally strongly electropositive) to the outermost shell of the other atom (generally strongly electronegative).
Formation of Sodium Chloride:
In the formation of sodium chloride, an electron is transferred from sodium atom to chlorine atom and a positive ion of sodium and negative ion of chlorine is formed. The resulting Na+ and Cl– ions, possessing configuration of neon (2,8) and argon (2,8,8) respectively combine to form an electrovalent compound as shown below:
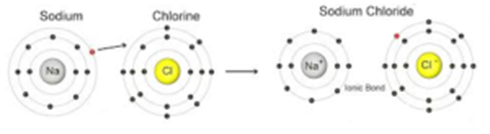
(2,8,1) (2,8,7) (2,8) (2,8,8)
Formation of Magnesium Chloride:
In the formation of magnesium chloride, two electrons are transferred from magnesium atom to two chlorine atom (one each) and positive ion of magnesium and two negative ions of chlorine is formed. The resulting Mg++ and two Cl– ions, possessing configuration of neon (2,8) and argon (2,8,8) respectively combine to form an electrovalent compound (MgCl2) as shown below:
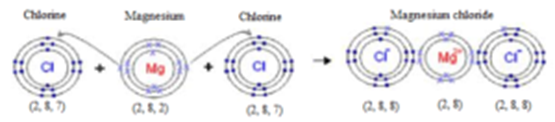
In the above examples, each ion has attained an octet in the outermost shell. When positive and negative ions come closer to each other, they are held by electrostatic forces of attraction.
Covalent Bond:
A bond established between two identical or different atoms by sharing one or more pairs of electrons is known as a covalent bond. Each atom contributes one electron to form a common pair i.e. the equal contribution of electrons followed by equal sharing. If one electron pair is shared, it is known as a single covalent bond. If two electron pairs are shared, it is a double covalent bond and so on.
It is observed in diatomic molecules like chlorine (CI2), oxygen (O2), hydrogen(H2), nitrogen (N2), fluorine (F2) etc. and other molecules like methane (CH4), water (H2O), carbon dioxide(CO2) etc

Formation of Chlorine Molecule:
Consider formation of chlorine molecule. Each atom of chlorine consists of seven valence electrons in outermost shell. The two atoms share one pair of electrons between them completing octet of each. One shared pair constitutes a single bond.
Cl + Cl → Cl – Cl i.e. Cl2
(2,8,7) (2,8,7) (2,8,8) (2,8,8)
Chlorine atoms Shared pair

Formation of Oxygen Molecule:
- Consider formation of oxygen molecule. Each atom of oxygen consists of six valence electrons in outermost shell. The two atoms share two pairs of electrons between them completing octet of each. two shared pairs constitutes a double bond.
O + O → O = O i.e. O2
(2,6) (2,6) (2,8) (2,8)
Oxygen atoms Shared two pairs

Formation of Nitrogen Molecule:
Consider formation of nitrogen molecule. Each atom of nitrogen consists of five valence electrons in outermost shell. The two atoms share three pairs of electrons between them completing octet of each. three shared pairs constitutes a triple bond.
N + N → N ≡ N i.e. N2
(2,5) (2,5) (2,8) (2,8)
Nitrogen atoms Shared three pairs

Coordinate Bond:
Coordinate bond is a special type of covalent bond When one atom has complete octet with the unshared pair of electrons (lone pair) and another atom is in need of a pair of electrons then they Form bond what is known as a coordinate bond. Both the electron required for sharing between two atoms are contributed by one atom only. The atom contributing the lone pair is known at Donor and the atom in need of two electrons is known as Acceptor.
Coordinate bond is represented by → from donor to the acceptor.
In ammonia nitrogen has a complete octet with a lone pair of electrons. It can donate the lone pair to hydrogen ion forming NH4+ ion.

Difference Between Covalent Bond and Co-ordinate Bond:
A bond established between two identical or different atoms by sharing of one or more pairs of valence electrons in the outermost shell is known as covalent bond while the coordinate bond is a special type of covalent bond in which only a single atom whose octet is complete contributes both the electrons required for sharing between two atoms.
In the formation of a covalent bond, each atom contributes one electron to form a common pair. i.e. the equal contribution of electrons followed by equal sharing, while in the formation of coordinate bond in which only a single atom whose octet is complete contributes both the electrons required for sharing between two atoms.
Inadequacies or Limitation of Octet Theory: (Need of Valence Bond Theory)
Failure to Explain Incomplete and expanded Octets:
It fails to explain the formation of ions and compounds with incomplete as in BeCI2 and BCI3 or expanded octet as in PCI5 and SF6 Octet rule in not obeyed in these cases, still, compounds are formed.

In the above structures, neither the octet of Be (4 – electrons around Be) nor of B (6 – electrons around B) is completed. These atoms have incomplete octets, however, they are stable compounds. The octet theory is unable to explain this phenomenon.
The central atoms phosphorus and sulphur have ten and twelve electrons around them respectively. Such molecules are said to have expanded octet. Octet rule in not obeyed in these cases, still compounds are formed.
Failure to explain the Nature of interacting forces:
The octet rule cannot explain the nature of the forces of interaction between two combining atoms. (There are attractive forces between the nucleus of one atom and electrons of other atom and there are repulsive forces between nucleus-nucleus and electron-electron of two atoms).

Octet theory fails to explain the nature of interacting forces it can be explained as follows
- the octet theory simply says that a covalent bond will be formed by sharing of electrons but tells nothing about the interacting forces.
- There are attractive forces between the nucleus of one atom and electrons of other atom and there are repulsive forces between nucleus-nucleus and electron-electron of two atoms.
- Octet theory does not explain what forces make the atoms to combine with each other.
Failure to explain Energy and reactivity:
It does not explain the energy relations of atoms and molecules. The molecule has lower energy and greater stability than the combining atoms. The molecule has lower energy and greater stability than the combining atoms. Octet theory fails to explain the energy evolved in bond formation and reactivity of molecules formed.

The energy given off or released per mole during the formation of a covalent bond is called bond energy. Greater the bond energy, the more stable will the bond and less reactive will be the molecule. The octet theory does not enable us to calculate the bond energy, in a chemical bond.
Octet theory fails to explain the reactivity of covalent molecules. Once the octet of all involved atoms is completed, the molecule should be as unreactive as an inert gas. Octet theory fails to explain the energy and reactivity of the resulting molecule. It can be explained as follows
Failure to explain the Geometry of the molecules:
It fails to explain the geometry of the molecule. Molecules have different shapes such as linear, trigonal, tetrahedral etc. Octet theory fails to explain the geometry of the resulting molecule. It can be explained as follows
- Molecules have different shapes such as linear, trigonal, tetrahedral, etc.
- The octet theory provides an idea about the number of covalent bonds formed but not the geometry of the molecules formed.
- Octet theory fails to explain the tetrahedral structure of methane, the distorted tetrahedral structure of ammonia and water, the trigonal planar structure of boron trifluoride and the linear structure of beryllium difluoride.
- BeF2 is a linear molecule while H2O is a v-shaped molecule. The octet theory fails to explain this difference.
Octet theory is only a guideline for writing “dot and dash” structures
Thus octet theory has a number of limitations and therefore another theory (valence bond theory) is required to explain the nature of the chemical bond.
Dot and Dash Structures Using Octet Theory:
Electrons are generally represented by a dot (.) or cross (x) and a covalent bond is expressed by a dash (-). The system of representing the formula of a compound using dot and dashes is called “Electron dot and dash formula”.
