Science > Chemistry > Electrochemistry > Reference Electrodes
The electrode whose potential is arbitrarily fixed or is exactly known at a given constant temperature is known as a reference electrode. Using reference electrodes unknown potential of any other single electrode can be found out e.g. two commonly used reference electrodes are standard hydrogen electrode (SHE) and Calomel electrode.
Use of Reference Electrodes:
A cell is constructed using the given electrode and reference electrode. Using a potentiometer and standard cell (like Weston cell) the e.m.f. of the cell can be measured. By knowing the e.m.f. of the cell and potential of the reference electrode, the potential of the electrode in question can be easily determined.
Standard Hydrogen Electrode (SHE).
SHE is defined as the electrode in which pure and dry hydrogen gas is bubbled at 1 atmospheric pressure and 298 K on a platinized platinum foil through a solution containing H+ ions at unit activity.
Construction:
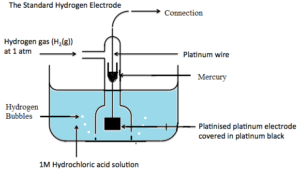
SHE consists of a glass jacket which has a small inlet at the top and many outlets at the bottom. Inside the glass jacket, there is a glass tube closed at both ends. It has a platinum wire sealed in it. At the lower end of the platinum wire, there is a platinized platinum plate. At the bottom of the glass tube, there is little mercury which is meant for good electrical contact. The glass jacket along with a glass tube is dipped in a vessel containing 1 M HCI solution.
Working:
Pure and dry hydrogen gas is bubbled through HCI solution from the inlet at a constant pressure of 1 atm. Hydrogen gas is adsorbed on the platinum plate and acts as a hydrogen electrode. An equilibrium between H2 gas and H+ ion is established across the metal.
Electrode reaction:
The electrode is reversible with respect to hydrogen ions. During working, hydrogen gas from platinum plate changes into hydrogen ions and electrons are set free. These electrons accumulate on the platinum plate.
If the electrode is serving as an anode, then the half-cell reaction is
H2(g) → 2H+(aq) + 2e– (oxidation)
The electrons set free remains on the platinum plate and transferred to the other electrode through Pt. wire. As the process is oxidation, a positive potential is developed. It is comparatively very small, it is arbitrarily taken as a zero.
If the electrode is serving as a cathode, then the half-cell reaction is
2H+(aq) + 2e– → H2(g) (reduction)
Representation of electrode:
When acting as an anode, Pt| H2(g) (1 atm.)| H+(aq) (1 M)
When acting as cathode, H+(aq) (1 M) | H2(g) (1 atm.) | Pt
Advantages of SHE:
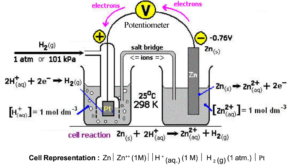
- SHE is used as a reference electrode. When it is coupled with any other electrode whose potential is to be determined. The potential of the cell is then measured using a potentiometer. Since the potential of SHE is zero, the potential of the cell is equal to the potential of another electrode or e.m.f. of the cell itself. Thus when SHE is used, the correction for its own potential is not necessary.
- It can be used over the entire pH range.
- It gives no salt error.
- It consists of a pH scale with voltage measurement.
Difficulties in setting up of SHE:
- It is difficult to obtain 100 % pure and dry hydrogen gas. Even traces of impurities in the hydrogen gas makes the electrode inactive and irreversible.
- It is difficult to maintain exactly 1 atmospheric pressure on hydrogen gas for a longer time.
- It is difficult to maintain the concentration of HCI solution as 1 M because due to the bubbling of hydrogen gas through HCI solution, water is evaporated and hence the concentration of HCI solution may change.
- Since it is made up of glass, it is not so handy.
- Platinum used is rather expensive.
- It is difficult to prepare ideal platinized platinum.
Arrangement to Find Oxidation Potential of another Electrode Using SHE:
Calomel Electrode:
Construction:
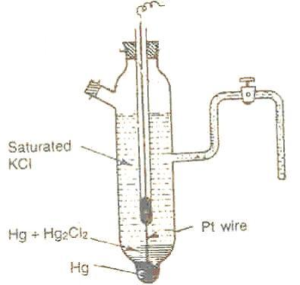
The calomel electrode consists of a broad glass tube having sidearm as shown in the figure. The sidearm is used for dipping it any solution used for coupling the calomel electrode. At the bottom of the glass tube, there is pure mercury and a platinum wire is sealed into it at the bottom for electrical connections. The wire runs through a separator glass tube to the top of the tube for electrical contact. Above pure mercury, there is a paste of mercurous chloride (calomel) (Hg2Cl2) in mercury. The rest of the glass vessel and sidearm A is filled with a saturated KCl solution. KCI solution of 0.1 M or of 1 M can also be used. Sidearm is plugged with glass wool. The glass tube is closed from the top.
Working:
Since the calomel electrode is reversible, two types of reactions are possible depending upon the nature of another electrode with which it is coupled.
When acting as negative electrode:
2 Hg(l) → 2 Hg+ + 2 e–
2 Hg+ + 2 Cl – → Hg2Cl2(s)
The net oxidation reaction is
2Hg(l) + 2Cl–(sat) → Hg2Cl2(s)+ 2e–
Thus oxidation takes place when it is coupled with other electrode having lower oxidation potential.
When acting as positive electrode:
Hg2Cl2(s) → 2Hg(l) + 2Cl––
2 Hg+ + 2 e– → 2 Hg
The net reduction reaction is
Hg2Cl2(s) + 2 e– → 2 Hg+ + 2Cl–
Thus reduction takes place when it is coupled with other electrode having greater oxidation potential.
Representation of Electrode:
When acting as anode: Pt | Hg(l) | Hg2Cl2(s) | KCl(sat)
When acting as anode: KCl(sat) | Hg2Cl2(s) | Hg(l) |Pt
Oxidation Potential of Calomel Electrode:
The oxidation potential of the calomel electrode depends upon the concentration of KCl solution used. The negative potentials indicate that when combined with SHE reduction takes place at the calomel electrode.

Advantages of calomel electrode:
- It is easy to set up and easily reproducible.
- It is convenient and easy to transport.
- It is very compact and smaller in size requires little space.
- No separate salt bridge is required as it has already a side tube containing KCl solution.
- Potential does not change appreciably with time and a slight change in temperature.
Disadvantages of Calomel Electrode:
- When half-cell potentials are to be measured, compensation for potential is necessary.
- The calomel electrode cannot be used in the measurement of potentials of the cell where K+ and Cl – ions interfere in the electrochemical reactions of the cell.
- The oxidation potential of the electrode depends on the concentration of KCl. If the concentration of KCl changes, the oxidation potential of electrode changes.
Previous Topic: Representation of Electrochemical cell
Next Topic: Types of Electrodes