Science > Chemistry > Electrochemistry > Representation of Electrochemical Cell
In this article, we shall study the representation of cell using conventions on paper. We shall also discuss the construction and working of the salt bridge.
Electrochemical (Voltaic or Galvanic) cell consists of two half cells. In one half-cell, an oxidation reaction takes place and electrons are generated in the process. While in the second half cell reduction reaction takes place and electrons are absorbed or consumed in this process. The two half-cells are connected to each other internally by porous partition and connected externally by means of connecting wire.
Both the oxidation and reduction reaction takes place simultaneously and separately. The movement of electrons from oxidation half-cell to reduction half-cell through external circuit constitutes the electrical current.
Convention for Representing the Voltaic Cell:
Every time it is not possible to draw neat diagrams of the cell. So as to avoid this difficulty, following conventions (rules) are used.
- The electrode with greater oxidation potential (negative electrode or anode) is written to the left. Here oxidation takes place.
- The electrode material is written first, followed by its electrolyte.
- The electrode with lower oxidation potential ( positive electrode or cathode ) is written to the right. Here reduction takes place.
- The electrolyte is written first followed by the electrode material.
- A single vertical line is drawn between the electrode and its electrolyte which represents direct contact but the separation of the two phases.
- The double vertical line is drawn between two salt solutions which indicate indirect contact by means of a porous pot or a salt bridge.
- Concentration or activity of solutions at two electrodes is written in brackets as (C = 1), (C = 2) or (a = 1), (a = 2).
- In the case of gas electrodes, inert metal conductors like platinum are used to establish electrical contact. It should be incorporated in the convention. e.g. a) Hydrogen electrode is represented as
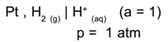
- The representation of the Daniell cell by the above convention is as follows.
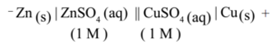
More Examples:
A cell consisting of zinc and cadmium electrodes:
As zinc has higher oxidation potential, it acts as -ve electrode. Cadmium acts as a +ve electrode. Oxidation takes place at zinc while reduction takes place at Cadmium.
– Z n | Z n++ || Cd++ | Cd +
(1 M) (1 M)
At anode (Zn) : Zn → Zn++ + 2 e– (oxidation)
At Cathode (Cd): Cd++ + 2 e– → Cd (reduction)
Net cell reaction : Zn + Cd++ → Zn++ + Cd (redox)
Representation of a cell consisting of hydrogen and copper electrodes:
As hydrogen has greater oxidation potential than copper, it acts as -ve electrode (anode) and copper act +ve electrode (cathode). Oxidation takes place at hydrogen and reduction takes place at copper.
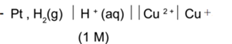
At anode (H2) : H2(g) → 2 H+ + 2– (oxidation)
At cathode (Cu) : Cu++ + 2 e– → Cu (reduction)
Net cell reaction : H2(g) + Cu++ → 2H+ + Cu (redox)
Representation of a cell consisting of copper and silver electrodes:
As copper has greater oxidation potential than silver, acts as anode and silver act as the cathode. Oxidation takes place at copper and reduction takes place at silver.
– Cu | Cu++ || Ag+ | Ag+
(C =1) (C = 2)
At anode (Cu): Cu → Cu++ + 2 e– (oxidation)
At cathode (Ag): 2 Ag+ + 2 e– → 2 Ag (reduction)
Net cell reaction: Cu + 2 Ag+ → Cu++ + 2 Ag (redox)
Representation of a cell from the cell reaction:
1/2 Cl2+ e– → Cl–
2 OH– → 1/2 O2 + H2O + 2e–
As chlorine undergoes reduction hence chlorine is a cathode and it must be written to the right. In the second reaction OH– ions undergo oxidation hence oxygen electrode is an anode and it must be written to the left. The cell can be represented as,
Pt, O2(g) | OH– (aq) || Cl–(aq) | Cl2(g) ,Pt
Salt Bridge:
It consists of an inverted U shaped glass tube containing the saturated solution of a strong electrolyte like KCl, KNO3, NH4NO3 immobilized by agar-agar gel with glass wool plugs at the two ends.

Working: The two electrolytic solutions in two half-cells are connected by dipping the arms of the tube in an inverted position in the solutions.
Electrodes and its Types:
Electrodes are metallic or non-metallic rods immersed in the electrolyte. They conduct electric current through them. Carbon and platinum are mainly used electrodes because they are inert and do not get dissolved in the electrolytic solution.
Electrodes are of two types, a) indicator electrode b) Reference Electrode
Indicator Electrode:
The electrode whose potential depends upon the concentration of a particular ion in the solution in which it is dipped and is usually used to find out the concentration of ions in the solution is known as indicator electrode.
It usually consists of metal in the form of wire or rod kept in contact with its salt solution.
e.g. Ag(s) | Ag+ (aq) (a = x M)
The electrode system is used for the determination of the concentration of the solution used in that half-cell. All electrodes except the reference electrode are called indicator electrodes.
Reference Electrode:
The electrode whose potential is arbitrarily fixed or is exactly known at a given constant temperature is known as a reference electrode. Using reference electrode unknown potential of any other single electrode can be found out e.g. Two commonly used reference electrodes are Standard hydrogen electrode (SHE) and Calomel electrode.
A cell is constructed using the given electrode and reference electrode. Using a potentiometer and standard cell (like Weston cell) the e.m.f. of the cell can be measured. By knowing the e.m.f. of the cell and potential of the reference electrode, the potential of the electrode in question can be easily determined.
Previous Topic: Secondary Electrochemical Cells
Next Topic: Reference Electrodes