Science > Chemistry > Atomic Structure > Rutherford’s Atomic Model
In this article, we shall study Thomson’s model of an atom and its deficiencies and Rutherford’s atomic model and its deficiencies.
Thomson’s Model of an Atom:
J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement. Many different names are given to this model, for example, plum pudding, raisin pudding, or watermelon. It can be visualized as a positive charge with plums or seeds (electrons) embedded into it (watermelon). An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom.
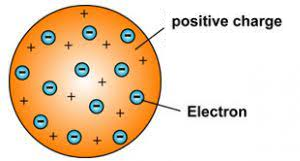
Although this model was able to explain the overall neutrality of the atom but was not consistent with the results of later experiments.
Basis of Rutherford’s Atomic Model:
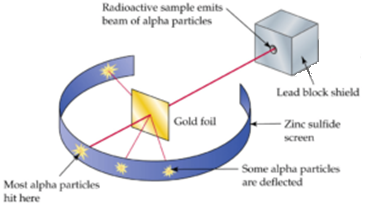
Alpha particles from a radioactive substance were made incident on the thin foil of gold of thickness 10-7 m. After passing through the foil, the alpha particles were detected at various places on the ZnS screen or photographic plate. The following four types of particles were detected.

- Most of the alpha particles just passed through without any deviation as if there is empty space.
- A few alpha particles were deflected through smaller angles.
- A few alpha particles deviated through larger angles.
- Very few rebounded back This larger deflection is possible only if α-particles collide with heavy and positively charged particles inside the atom because like charges only repel each other. This massive +ve charge is at centre of an atom and called nucleus.
- Very few alpha particles were rebounded i.e. they deviated through 180°. This concludes that the nucleus is very small as compared to the volume of the atom.
Conclusions :
- Based on the experiment Rutherford concluded that most of the alpha particles having positive charge went through the foil undeflected. Hence there must be empty space in the atom.
- Some of the particles were deflected. It is due to the positive charge present in a very small space within the atom. He called this centrally cored and positively charged region of an atom as a nucleus. The rest of the portion of the atom should be empty.
Rutherford’s Atomic Model:
From the observations of his experiment, Rutherford put forward the concept of his atomic model.
The atom consists of the centrally located positively charged nucleus. The whole mass of an atom is concentrated in the nucleus. Around the nucleus, there is empty space in which the negatively charged electrons revolve in different orbits. Rutherford’s model of an atom is also called as a planetary model of an atom.
Limitations Of Rutherford’s Atomic Model:
- The negatively charged electrons revolve around the nucleus in a circular orbit, hence they possess centripetal acceleration. According to the classical theory of electromagnetism, accelerated charge radiates energy continuously. Therefore, the
electron should radiate energy while going round the nucleus losing its energy continuously. therefore, it should approach nearer the nucleus while going round emitting radiations of increasing frequency and finally falling in the nucleus. Thus it should move in a spiral path and should emit continuous spectrum and thus structure atom is not stable. Actually, the spectrum observed is line spectrum of definite frequency, and hence a modification of Rutherford’s atom model was necessary.

- This model of an atom fails to explain the distribution of electrons in different orbit around the nucleus.
- According to Rutherford’s model of an atom, the atomic spectrum should be continuous. But atomic spectrum is found to be discontinuous. Rutherford model fails to explain the discontinuity of the atomic spectrum.
- This model also fails to explain the line spectra of atoms, which show discrete lines, each line corresponds to a fixed frequency.