Science > Chemistry > Physical Chemistry > Ionic Equilibria > Solubility Product
In this article, we shall study the concept of solubility, solubility product, and its applications.
Some ionic solids are highly soluble in water while others are almost insoluble in it. The solubility of ionic solid depends on lattice enthalpy of the salt and hydration enthalpy of ions in solution. The lattice enthalpy of salt is defined as the energy required to overcome the attractive forces between the ions. It is always positive. The hydration enthalpy or solvation enthalpy is the energy released during the interaction between the ions and solvent molecules. It is always negative. If salt is to be dissolved then its solvation enthalpy should be greater than its lattice enthalpy.
Solubility:
The concentration of a substance in its saturated solution is called as its solubility at a given temperature. It is denoted by letter ‘S’ It is expressed as grams per litre or as moles per litre at a given temperature.
Classification of Solids on the Basis of Solubility:
- The solids having a solubility greater than 0.1 M are classified as soluble solids e.g. NaCl, Sugar, etc.
- The solids having a solubility between 0.01 M and 0.1 M are classified as slightly soluble solids e.g. calcium phosphate.
- The solids having a solubility less than 0.01 M are classified as sparingly soluble solids e.g. barium sulphate, silver chloride, etc.
Sparingly Soluble Salt:
A certain substance like AgCl, PbSO4, BaSO4 etc. have negligible solubility in water at ordinary temperature. Such substances which are practically insoluble in water are called as sparingly soluble electrolytes. The amount of such salts getting dissolved is so small that their saturated solution may be regarded as extremely dilute and hence dissolved part can be considered as completely ionized.
Solubility Product:
In a saturated solution of a sparingly soluble electrolyte, the product of molar concentration of ions is constant at a given temperature. This constant ‘ Ksp ’ is called a solubility product.
Explanation:
Suppose ‘BA’ is a sparingly soluble electrolyte. In aqueous solution, it dissociates to a very little extent there exist two equilibria.
BA(s) → BA(aq) ⇌ B+(aq) + A–(aq)
The mass law equation of the equilibrium is
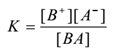
But [BA] = constant
∴ K . constant = [B+][A–]
∴ Ksp = [B+][A–]
Where Ksp is solubility product. If ‘S’ moles/dm3 is solubility of electrolyte ‘BA’ then [B+] = S and [A–] = S
Ksp = [S] [S]
Ksp = S2
| Salt Type | Relation with Ksp | Examples of salt |
| AB | Ksp = (s)(s) = s2 | AlPO4, AgCl, BaSO4, ZnS |
| AB2 | Ksp = (s)(2s)2 = 4s3 | PbCl2, HgCl2 |
| A2B | Ksp = (s) 2 (2s) = 4s3 | Ag2CrO4, Ag2C2O4, Ag2SO4 |
| AB3 | Ksp = (s) (3s)3 = 27s4 | Fe(OH) 3, Al(OH) 3, Cr(OH) 3 |
| A3B2 | Ksp = (3s) 3 (2s)2 = 108 s5 | Ca3(PO4)2, Zn3(PO4)2 |
| A3B4 | Ksp = (3s) 3 (2s)4 = 6912 s7 | Zn3(PO4) 4 |
| AxBy | Ksp = (xs) x (ys)y = xxyy sx+y |
Effect of pH on Solubility:
The solubility of salt of weak acids increases in more acidic solutions e.g. ZnS, CuS, NiS, etc. Marble (CaCO3) statues and monuments corrode by the effect of acid rain.
Saturation, Unsaturation and Precipitation:
- In a solution, when the ionic product is equal to the solubility product. Then the solution is just saturated and precipitation doesn’t occur.
- In a solution, when the ionic product is less than the solubility product then the solution is unsaturated and precipitation doesn’t occur.
- In a solution, when the ionic product exceeds the solubility product then the solution is supersaturated and precipitation of electrolyte takes place.
- Thus precipitation is possible only when the ionic product is greater than the solubility product.
Applications of Solubility Product:
In the precipitation of II group cations:
Group II cations like Cu++, Cd++, Pb++ etc. are precipitated as their sulphides.
Precipitation is carried out by adding dil. HCI followed by passage of H2S gas through the solution. Being weak acid, H2S ionizes as,
H2S(aq) ⇌ 2 H+ (aq.) + S– –(aq)
HCI being strong acid dissociate almost completely as,
HCl(aq) → H+(aq) + Cl–(aq)
The concentration of H+ is increased. Since H+ ions are common ions, due to common ion effect dissociation of H2S is suppressed so that S– – ion concentration is decreased to such an extent that only group II cations get precipitated. Ionic product of sulphides of II group cations exceeds solubility product, so only II group cations form a precipitate and other cations belonging to further groups remain as it is in the solution.
In the precipitation of III A group cations:
III A group cations like AI3+, Fe3+, Cr3+, etc. are precipitated as their hydroxides by adding NH4CI followed by adding NH4OH.
Being weak base NH4OH dissociate as
NH4OH(aq) ⇌ NH4+(aq) + OH–(aq)
Being strong electrolyte NH4CI dissociate as,
NH4Cl(aq) ⇌ NH4+(aq) + Cl–(aq)
Since NH4+ ions are common, their concentration increases and due to common ion effect dissociation of NH4OH is suppressed so that concentration of OH– ion decreases to such an extent that only IIIA group cations are precipitated. Ionic product of hydroxides of IIIA group cation exceeds solubility product while the ionic product of hydroxides of IIIB group is lower than solubility product hence only IIIA group cations are precipitated.
In Prediction of Precipitation:
Solubility product is the highest limit of ionic product at a particular temperature. When ionic product exceeds the solubility product, excess ions combine with each other to form the precipitate of the salt. Hence to find whether a precipitation can take place or not, ionic product of salt is calculated and it is then compared with the solubility product of the salt at the same temperature.
By knowing the molar concentration of ions in a solution and solubility product, it can be predicted whether precipitation would occur or not. Precipitation is an ionic reaction. According to the solubility product concept, precipitation occurs only when the ionic product exceeds solubility product. If Ksp = ionic product or Ksp > ionic product, then precipitation doesn’t occur.
In general
- Ionic product = Ksp, the solution is saturated (No precipitation)
- Ionic product < Ksp, the solution is unsaturated(No precipitation)
- Ionic product > Ksp, the solution is supersaturated (precipitation)
Example:
Ksp of BaSO4 at 298 K is 1 x 10-10 then for the precipitation of BaSO4 in the solution,
ionic product [Ba++ ] [SO4– –] > Ksp of BaSO4
[Ba++ ] [SO4– –] > 1 x 10-10