Science > Physics > Atoms, Molecule, and Nuclei > Rutherford’s Model of an Atom
In this article, we shall study Dalton’s atomic theory, Rutherford’s model of an atom, its merits, and demerits.
Dalton’s Atomic Theory:
This theory was proposed by English chemist John Dalton in 1808. The main propositions of the theory are as follows:
- Every element is made up of extremely small particles called an atom.
- The atoms are indivisible and they can neither be created nor be destroyed.
- Atoms of the same element resemble each other in all respects but differ from the atoms of other elements.
- Atom is the smallest unit of matter which takes part in a chemical reaction.
- When chemical compounds are formed they do so by the combination of atoms of different elements in the simple proportion of whole numbers.
- Atoms of different elements may combine in more than one proportion to form different compounds.
Fundamental Particles of an Atom:
Electrons:
- Electrons were discovered by British scientist Sir J.J. Thomson.in 1897 (Cathode ray tube experiment).
- The term electron was coined by Stoney.
- The charge on the electron was found by Milikan
- Specific charge ratio or charge to mass ratio or e/m ratio is obtained by J.J. Thomson.
Protons:
- Proton was discovered by E.Goldstein.
- They are located in the nucleus.
- They are positively charged particles.
Neutrons:
- Neutron was discovered by Chadwick in 1932.
- Neutrons have no charge i.e. they are electrically neutral.
- They are located in the nucleus of an atom.
Thomson’s Model of an Atom:
Thomson proposed this model in 1903. According to him, an atom consists of protons and electrons. The total number of protons is equal to the total number of electrons. Thus the net charge of the atom is zero. An atom consists of a positively charged sphere with negatively charged electrons embedded in it. This model of an atom is called the watermelon model or plum (dot) pudding model of an atom.
Geiger Marsden Experiment:
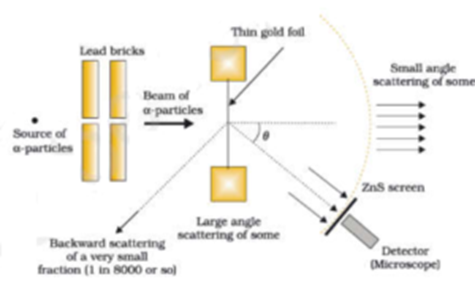
A narrow beam of alpha particles from the radioactive source was incident on a thin gold foil. The scattering of alpha particles takes place. The scattered alpha particles were detected by a detector fixed on a stand. The deviation of alpha particles from their original path is called the scattering angle. They observed that most of the alpha particles just passed through without any deviation as if there is empty space. A few alpha particles were deflected through smaller angles. A few alpha particles deviated through larger angles. This larger deflection is possible only if alpha-particles collide with heavy and positively charged particles inside the atom because like charges only repel each other. This massive +ve charge is at the centre of the atom and called the nucleus. Very few alpha particles were rebounded i.e. they deviated through 180°. This concludes that the nucleus is very small as compared to the volume of the atom.
Rutherford’s Model of an Aom:
From the observations of the above experiment, Rutherford put forward the concept of his atomic model. The atom consists of a centrally located positively charged nucleus. The whole mass of an atom is concentrated in the nucleus. Around the nucleus, there is empty space in which the negatively charged electrons revolve in different orbits. The total positive charge of the nucleus is equal to the total negative charge on orbiting electrons. Hence atom is electrically neutral. Rutherford’s model of an atom is also called as a planetary model of an atom.
Limitations of Rutherford’s Model of an Atom:
- The negatively charged electrons revolve around the nucleus in a circular orbit, hence they possess centripetal acceleration. According to the classical theory of electromagnetism, accelerated charge radiates energy continuously. Therefore, the electron should radiate energy while going around the nucleus losing its energy continuously. therefore, it should approach nearer the nucleus while going round emitting radiations of increasing frequency and finally falling in the nucleus. Thus it should move in a spiral path and should emit a continuous spectrum and thus structure atom is not stable. Actually, the spectrum observed is the line spectrum of definite frequency, and hence a modification to Rutherford’s atom model was necessary.
- This model of an atom fails to explain the distribution of electrons in different orbit around the nucleus. According to Rutherford’s model of an atom, the atomic spectrum should be continuous. But the atomic spectrum is found to be discontinuous. Rutherford’s model fails to explain the discontinuity of the atomic spectrum.
- This model also fails to explain the line spectra of atoms, which show discrete lines, each line corresponds to a fixed frequency.