Science > Chemistry > Electrochemistry > Types of Electrodes
In this article, we shall study different types of electrodes, their representation, writing cell reactions, and finding e.m.f. of a cell.
There are four types of electrodes
- Gas electrodes
- Metal–sparingly soluble metal salt electrodes
- Metal – metal ion electrodes
- Redox Electrodes
Gas Electrodes:
A gas electrode consists of a gas (e.g. H2, Cl2, O2) in contact with a solution containing the ions derivable from the gas e.g. H+, Cl-, OH-. The potential of the gas electrode depends upon the concentration of its ions in the solution and the pressure of a gas.
A gas electrode consists of gas, bubbled about inert metal wire (platinized platinum electrode) immersed in a solution containing ions with which gas is irreversible. Platinum is used as conductor and to adsorb the gas. e.g. Standard hydrogen electrode.
Examples of gas Electrodes:
Standard Hydrogen Electrode (SHE):
SHE is represented as,
Pt| H2(g) (1 atm.)| H+(aq) (1 M)
The half cell reactions are
H2(g) → 2H+(aq) + 2e– (oxidation) (L.H.S.)
2H+(aq) + 2e– → H2(g) (reduction) (R.H.S.)
The electrode potential is arbitrarily assigned zero. This electrode is cation electrode.
Chlorine gas electrode:
This electrode is anion electrode. Chlorine gas electrode is represented as,
Pt| Cl2(g) (1 atm.)| Cl–(aq) (1 M)
The half cell reactions are
2Cl–(aq) → Cl2(g) + 2e– (oxidation) (L.H.S.)
Cl2(g) + 2e– → 2Cl–(aq) (reduction) (R.H.S.)
Oxygen gas electrode:
Oxygen gas electrode is represented as,
Pt | O2(g) (1 atm)| OH– (aq) (1M)
The half cell reaction is
4OH– → 2H2O+ O2(g) + 4e– (oxidation) (L.H.S.)
2H2O + O2(g) + 4e– → 4OH– (reduction) (R.H.S.)
Metal-Sparingly Soluble Metal Salt Electrode:
Reversible anion electrode is also called as metal- sparingly soluble metal salt electrode. In this electrode a metal, a sparingly soluble salt of the metal in equilibrium with a solution containing the same anion as the sparingly soluble salt. e.g. Calomel electrode.
Metal – Metal Ion Electrodes:
In this case, the metal strip is kept in contact with the solution of a water-soluble salt-containing cation of the same metal.
e.g. Zn(s) | Zn++(aq)
In the electrochemical cell, the electrode having higher oxidation potential undergoes oxidation and acts as the anode or negative electrode and the electrode having lower oxidation potential undergoes reduction and acts as the cathode or positive electrode.
Examples of metal – metal ions electrodes:
Zn(s) | Zn++(aq)
Zn(s) → Zn++(aq) + 2e– (Oxidation)
Zn++(aq) + 2e– → Zn(s) (Reduction)
Cu(s) | Cu++(aq)
Cu(s) → Cu++(aq) + 2e– (Oxidation)
Cu++(aq) + 2e– → Cu(s) (Reduction)
Redox Electrode:
In these electrodes, an inert metal like Pt is dipped in a solution containing ions of an active metal in two different oxidation states.
Pt | Fe2+, Fe3+
Fe2+ → Fe3+ e– (Oxidation)
Fe+++ + e– → Fe++ (Reduction)
Pt | Sn2+, Sn4+
Sn2+ → Sn4+ + 2e– (Oxidation)
Sn4+ + 2e– → Sn2+ (Reduction)
Writing Cell Reaction and Finding E.M.F. of a Cell:
Redox Potential:
The potential developed due to the ability of ions to lose or gain electrons forming a higher or lower stable oxidation state is called redox potential.
The redox potential depends upon the ratio of concentrations of two types of ions.
Pt | Fe2+(aq)(1M), Fe3+ (aq) (1M) E2ox= – 0.771 V
Representation of cells containing standard and reference electrodes:
A cell composed of zinc rod contact with 1 molar zinc ion solution and saturated calomel electrode.
Zn(s)| Zn2+(1M) || KCl(aq) (saturated) | Hg2Cl2(s)|Hg(l), Pt +
Cell composed of SHE and saturated calomel electrode
Pt | H2(g) (1 atm)| H+(aq) (1M) || KCl(aq)(saturated)|Hg2Cl2(s)| Hg(l) ,Pt +
Cell Reactions:
Steps to Write Cell Reaction of Galvanic Cell:
- Represent the given galvanic cell with standard convention.
- The electrode on the left side of the representation shows that it is anode and oxidation takes place at this electrode. Write half cell oxidation reaction half-cell reaction for it.
- The electrode on the right side of the representation shows that it is cathode and reduction takes place at this electrode. Write a half-cell reduction reaction half-cell reaction for it.
- Balance above two reactions for electrons for oxidation and reduction reaction.
- Add the two reactions and obtain net (overall) cell reaction.
Step – 1: Represent the cell conventionally:
Pb(s) | Pb2+(aq) (1M) || Ag+(aq) (1M)| Ag(s) +
Step – 2: Write left hand side half-cell reaction: Pb(s) is on the left side of the representation shows that it is anode and oxidation takes place at Pb(s) electrode.
Pb(s) → Pb2+(aq) + 2e– (Oxidation) … (1)
Step – 3: Write right hand side half-cell reaction: Ag(s) is on right side of the representation shows that it is cathode and reduction takes place at Ag(s) electrode.
Ag+(aq) + e– → Ag(s) (Reduction) … (2)
Step – 4: Balance the Electrons of above two half cell reactions:
Multiply equation (2) by 2 to balance electrons.
2Ag+(aq) + 2e– → 2Ag(s) (Reduction) … (2)
Step – 5: Adding equations (1) and (3) we get overall reaction.
Pb(s) + Ag+(aq) → Pb2+(aq) + Ag(s)
Steps to Find E.M.F. of Galvanic Cell:
- Represent the given galvanic cell with standard convention.
- The electrode on the left side of the representation shows that it is anode and oxidation takes place at this electrode.
- The electrode on the right side of the representation shows that it is cathode and reduction takes place at this electrode.
- Obtain standard oxidation potential values from the electromotive series for the material of cathode and anode.
- Use the following formula for calculation of e.m.f. of a cell.
EoCell = Eo(ox/cathode) – Eo(ox/anode)
OR
EoCell = Eo(ox/cathode)+ Eo(red/anode)
To find e.m.f. of Daniel Cell :
Step – 1: Represent the cell conventionally
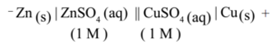
Step – 2: Decide anode and cathode: Pb(s) is on the left side of the representation shows that it is anode and oxidation takes place at Pb(s) electrode. Ag(s) is on the right side of the representation shows that it is cathode and reduction takes place at Ag(s) electrode.
Step – 3: Get values of oxidation potential or reduction potential for electrodes from From electrochemical series
Eo(ox/Zn) = 0.76 V and EEo(ox/Cu) =-0.34 V
Step – 4: calculate e.m.f of cell:
EoCell = Eo(ox/cathode) – E(ox/anode)
EoCell = Eo(ox/Zn) – Eo(ox/Cu)
EoCell = 0.76 – (- 0.34)
EoCell = 0.76 + 0.34
EoCell = 1.1 V
Previous Topic: Reference Electrodes
Next Topic: Nersnt Theory of Electrode Potential
Next Topic:
3 replies on “Types of Electrodes”
That’s was so great
Good presentation.
Nice examples