Science > Chemistry > Electrochemistry > Use of Nernst Equation
In this article, we shall study the use of the Nernst equation to find e.m.f. of cell and electrodes.
Convention Followed While Calculation of Cell Potential (e.m.f.):
- In the symbolic representation of the cell, the right-hand side electrode is the cathode (positive electrode) and the left-hand side is the anode (negative electrode).
- All standard potentials are reduction potentials that are they refer to a reduction reaction.
- The cathode has a higher standard potential than the anode.
- For spontaneous reaction to take place the cell potential should be positive.
Illustrations for Use of Nernst Equation:
When Reactions are given:
Example – 1:
Cr(s) + 3Fe3+ (aq) → Cr3+(aq) + 3Fe2+ (aq)
The cell formation is
Cr(s)| Cr3+(aq)|| Fe2+(aq),Fe 3+(aq)| Pt
The half cell reactions are
Cr(s) → Cr3+(aq)+ 3e– (Oxidation)
Fe3+(aq)+ 3e– → Fe2+(aq)(Reduction)
Hence n = 3
Nernst equation is
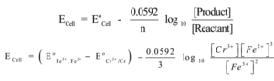
Example – 2:
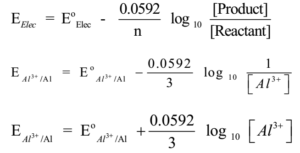
Al3+(aq) + 3e- → Al(s) ,
Here n = 3, Nernst equation is
When Type of Electrode is Given:
Redox Electrode:
Example – 1:
Pt | Sn2+, Sn4+
The Reduction reaction is
Sn4+(aq)+ 2e– → Sn2+(aq)(Reduction),
Hence n = 2, Nernst equation is
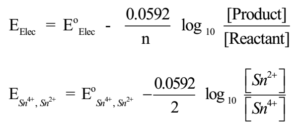
Example – 2:
Pt | Fe2+, Fe3+
The Reduction reactions are
Fe3+(aq) + 1e– → Fe 2+(aq)(Reduction)
Hence n = 1, Nernst equation is

Metal Metal Ion Electrode:
Example – 1:
Zn(s)| Zn++(aq)
Reduction reaction for it is
Zn(s) → Zn++(aq) + 2e–
Here n = 2, Nernst equation is

Example – 2:
Al(s)| Al3+(aq)
The reduction reaction is
Al3+(aq) + 3e- → Al(s)
Here n = 3, Nernst equation is

Metal Sparingly Soluble Salt Electrode:
Example – 1:
Cl– (aq) | AgCl(s)| Ag
The Reduction reaction is
AgCl(s)+ e– → Cl– (aq) + Ag(s) (Reduction)
Hence n = 1, Nernst equation is

Gas Electrode:
Example – 1:
Cl– (aq) | Cl2(g), (1 atm)| Pt
The Reduction reaction is
½ Cl2(g) + e – → Cl– (aq) (Reduction)
Hence n = 1, Nernst equation is

Important Terms:
Half-Cell:
An electrode in contact with an electrolyte containing its own ions is called a half cell. e.g. In Daniel cell, the zinc rod dipped in zinc sulphate solution is called zinc half cell.
Half-Cell Reaction:
The reaction taking place in a half cell or reaction taking place at each electrode is called half-cell reaction. e.g. In Daniel cell in zinc half cell oxidation takes place. Therefore the half-cell reaction is
Zn(s) → Zn++(aq) + 2e–
Cell:
A combination of two half-cells such that oxidation takes place at one half cell and reduction takes place at other half-cell is called the cell. e.g. A Daniel cell is formed by the combination of zinc half cell and copper half cell. Oxidation takes place at zinc half cell and the reduction takes place at the copper half cell.
Single Electrode Potential:
The difference of potential between the electrode and its salt solution around it at equilibrium is called a single electrode potential. Electrode potential depends upon
- Nature of the element/ metal,
- Concentration or activity of ions in solution
- Temperature and
- Pressure in case of gas.
Standard Electrode Potential (E°):
The difference of potential between the electrode and its salt solution around it containing ion concentration at a unit activity at 298 K is called standard electrode potential.
Oxidation Electrode Potential (Eox):
The difference of potential between the electrode and its salt solution around it at equilibrium and at constant temperature due to oxidation is called oxidation potential.
Standard Oxidation Potential (E°ox):
The difference of potential between the electrode and its salt solution around it containing ion concentration at a unit activity at 298 K due to oxidation is called standard oxidation potential (S.O.P.).
Reduction Electrode Potential (E°red):
The difference of potential between the electrode and its salt solution around it at equilibrium and at constant temperature due to reduction is called reduction potential.
Standard Reduction Potential (E°red):
The difference of potential between the electrode and its salt solution around it containing ion concentration at a unit activity at 298 K due to reduction is called standard reduction potential (S.R.P.).
Standard e.m.f. of Cell:
The algebraic sum of the standard oxidation potential of one electrode (anode) and the standard reduction potential of another electrode (cathode) is called the standard e.m.f. of a cell.

Note:
The oxidation potential of electrode is equal to the reduction potential of the electrode with the opposite sign

Gibb’s Energy Change:
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the maximum or reversible work that may be performed by a thermodynamic system at a constant temperature and pressure (isothermal, isobaric).
As the cell reaction in an electrochemical cell progresses, electrons move through a wire connecting the two electrodes until the equilibrium point of the cell reaction is reached, at which point the flow of electrons ceases. Just cell performs the work. In electrochemistry, the maximum amount of electrical work a galvanic cell can do at constant temperature and pressure is Gibb’s free energy.
The amount maximum work a galvanic cell can do is given as
Electrical work = Amount of charge (nF) × Cell potential (Ecell)
Electrical work = n F Ecell
The reversible electrical work done in a galvanic cell reaction is equal to the decrease in its Gibb’s energy
Thus, Electrical work = – ΔG
∴ – ΔG = n F Ecell
∴ ΔG = – n F Ecell
The standard Gibb’s energy change is given by
ΔG° = – nFE°cell
Gibb’s energy is an extensive property, which depends on the amount of substance. But the electrical potential is an intensive property which does not depend on the amount of substance. Thus E°cell remains constant. Thus if ΔG° changes there is the corresponding change in the number of electrons. It can be explained as follows
ΔG° = – n F E°cell

If the stoichiometric equation of redox reaction is multiplied by 2, then the standard Gibb’s energy ΔG° gets doubled and the number of electrons ‘n’ also gets doubled.

From (1) and (2) we can see that the e.m.f. of cell in both cases is the same. It shows that electrical potential is an intensive property that does not depend on the amount of substance.
Relation between Standard Cell Potential and Equilibrium Constant:
The Gibb’s free energy of a galvanic cell is given by
G° = – n F E°cell
By thermodynamical and equilibrium concept

Previous Topic: Nernst Theory of Electrode Potential
Next Topic: Electrochemical Series and its Applications