Science > Physics > Atoms, Molecule, and Nuclei > Hydrogen Spectrum
The origin of spectral lines in the hydrogen atom (Hydrogen Spectrum) can be explained on the basis of Bohr’s theory. The hydrogen atom is said to be stable when the electron present in it revolves around the nucleus in the first orbit having the principal quantum number n = 1. This orbit is called the ground state.
The electron gains energy from the surrounding and jumps into a higher orbit with principal quantum number n = 2, 3, 4, 5, ….. These higher orbits are called excited states. When electrons start revolving in the excited state the atom becomes unstable. To acquire stability the electron jumps from the higher orbit to lower orbit by the emission of the energy of value hν. Where ν is the frequency of radiation energy or radiation photon. This radiation is emitted in the form of spectral lines.
The Expression for the Wavelength of a line in the Hydrogen Spectrum:
Let En and Ep be the energies of an electron in the nth and pth orbits respectively (n > p) So when an electron takes a jump from the nth orbit to the pth orbit energy will be radiated in the form of a photon or quantum such that
En – Ep = hν ………… (1)
where ν is the frequency of radiation, h = Planck’s constant
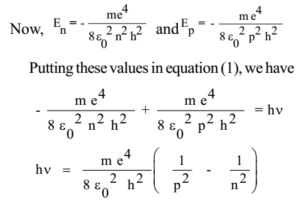

This formula is called Bohr’s formula of spectral lines.
The wavelength λ obtained is characteristic wavelength due to jumping of the electron from nth orbit to pth orbit. We get different series of spectral lines due to the transition of the electron from different outer orbits to fixed inner orbit.
Energy Level Diagram for Hydrogen Atom:
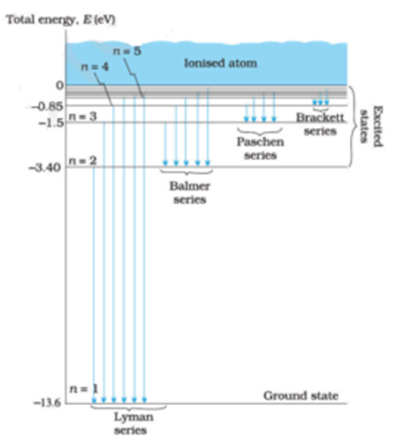
Energy level diagrams indicate us the different series of lines observed in a spectrum of the hydrogen atom. The horizontal lines of the diagram indicate different energy levels. The vertical lines indicate the transition of an electron from a higher energy level to a lower energy level.
It is very important that as indicated in the diagram each transition corresponds to a definite characteristic wavelength. Thus different transitions give different series of lines. Different Series obtained are a) Lyman series, b) Balmer series, c) Paschen series, d) Brackett series, e) Pfund series and f) Henry series
Different Series in Hydrogen Spectrum:
Lyman Series:
If the transition of electron takes place from any higher orbit (principal quantum number = 2, 3, 4,…….) to the first orbit (principal quantum number = 1). We get a Lyman series of the hydrogen atom. It is obtained in the ultraviolet region.

This formula gives a wavelength of lines in the Lyman series of the hydrogen spectrum. Different lines of Lyman series are
- α line of Lyman series p = 1 and n = 2
- α line of Lyman series p = 1 and n = 3
- γ line of Lyman series p = 1 and n = 4
- the longest line of Lyman series p = 1 and n = 2
- the shortest line of Lyman series p = 1 and n = ∞
Balmer Series:
If the transition of electron takes place from any higher orbit (principal quantum number = 3, 4, 5, …) to the second orbit (principal quantum number = 2). We get Balmer series of the hydrogen atom. It is obtained in the visible region.

This formula gives a wavelength of lines in the Balmer series of the hydrogen spectrum. Different lines of Balmer series area l
- α line of Balmer series p = 2 and n = 3
- β line of Balmer series p = 2 and n = 4
- γ line of Balmer series p = 2 and n = 5
- the longest line of Balmer series p = 2 and n = 3
- the shortest line of Balmer series p = 2 and n = ∞
Paschen Series:
If the transition of electron takes place from any higher orbit (principal quantum number = 4, 5, 6, …) to the third orbit (principal quantum number = 3). We get Paschen series of the hydrogen atom. It is obtained in the infrared region.

This formula gives a wavelength of lines in the Paschen series of the hydrogen spectrum.
Brackett Series:
If the transition of electron takes place from any higher orbit (principal quantum number = 5, 6, 7, …) to the fourth orbit (principal quantum number = 4). We get the Brackett series of the hydrogen atom. It is obtained in the far-infrared region.

This formula gives a wavelength of lines in Brackett series of the hydrogen spectrum
Pfund Series:
If the transition of electron takes place from any higher orbit (principal quantum number = 6,7, 8, …….) to the fifth orbit (principal quantum number = 5). We get Pfund series of the hydrogen atom. It is obtained in the far-infrared region.

This formula gives a wavelength of lines in the Pfund series of the hydrogen spectrum
Notes: Shortest wavelength is called series limit
Continuous or Characteristic X-rays:
Characteristic x-rays are emitted from heavy elements when their electrons make transitions between the lower atomic energy levels. The characteristic x-ray emission which is shown as two sharp peaks in the illustration at left occurs when vacancies are produced in the n=1 or K-shell of the atom and electrons drop down from above to fill the gap.

The x-rays produced by transitions from the n=2 to n=1 levels are called K-alpha x-rays, and those for the n=3 to n = 1 transition are called K-beta x-rays. For a particular material, the wavelength has definite value. Hence these x rays are called continuous or characteristic X-rays. The values of energy are different for different materials.
The frequencies of the characteristic x-rays can be predicted from the Bohr model. Moseley measured the frequencies of the characteristic x-rays from a large fraction of the elements of the periodic table and produced a plot of them which is now called a “Moseley plot”.
Characteristic x-rays are used for the investigation of crystal structure by x-ray diffraction. Crystal lattice dimensions may be determined with the use of Bragg’s law in a Bragg spectrometer.
6 replies on “Hydrogen Spectrum”
very nice notes
For any student who is a logical thinker ( and presumably works from first principles ) plucking a formula from thin air using memory work is not helpful. I expect that physics degree examinations are still based largely on memory work and give those who have a poor memory little hope.
Being a logical thinker left me with a 3rd but made me into a teacher who explained things in depth.
My son gained a 1st in Physics since he has a good memory but today my application of physics outstrips his but he has been rewarded more in his career.
Nice ,
That’s a cool and fantastic write up
We thank u for ur support to our study
Such a comprehensive and convincing extract.
More blessing.