Science > Physics > Heat Transfer > Convection and Radiation
In this article, we shall study different modes of heat transfer.
Convection:
Convection is a mode of heat transfer through a material medium in which heat energy is carried from one place to another by actual motion “migration” of heated matter.
Consider a beaker containing water small quantity of sawdust in added to this water. The beaker is then heated it was found that the sawdust particles start moving from bottom to top and then from top to bottom in a circular way.
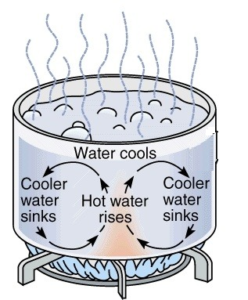
When we heat the liquid in the vessel the particles at the lower level get heated first hence there is an expansion of water at that layer. Hence the density of water at that layer decreases this lowe density water starts rising upward to float and the cold water with the higher density moves downward this process continue until the boiling point of water.
Phenomena Associated with Convection:
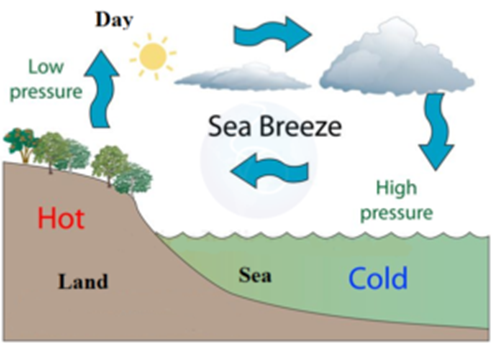
Sea Breeze:
During the daytime, the landmass having higher sp. heat gets were heated up than the seawater having lower sp. heat the air near landmass will get more heated up (since its specific heat is higher than water) than that near seawater the heated air near landmass having low density starts. Rising up thus creating low pressure gone on the landmass and thus the wind starts, blowing from sea i.e. high-pressure region to land i.e. low-pressure region these winds are called sea breezes.
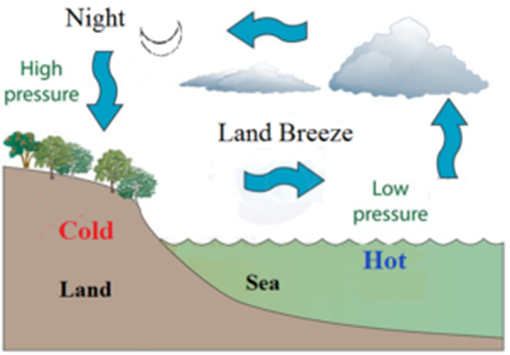
Land Breeze:
During night time the landmass having higher sp. heat loses, heat faster than the seawater having less sp. heat. The air near the surface of seawater will get more heated up than that near landmass. The more heated air near seawater rises upward due to low density. Thus a low-pressure zone is created on seawater. Thus wind starts blowing from land having high-pressure region to sea having low-pressure region. These winds are called the land breeze.
Trade Wind:
Trade wind is the steady surface wind on the earth blowing in from north-east towards the equator.

The equatorial and polar regions of the earth receive unequal solar heat. Air at the earth’s surface near the equator is hot while the air in the upper atmosphere of the poles is cool. A convection current would be set up, with the air at the equatorial surface rising and moving out towards the poles, descending and streaming in towards the equator.
Due to the rotation of the earth, modifies the direction of a convection current. Because of the rotation of the earth air close to the equator has an eastward speed of 1600 km/h, while it is zero close to the poles. As a result, the air descends not at the poles but at 30° N (North) latitude and returns to the equator. This flow of wind is called the trade wind.
Other Examples:
- The exhaust fans, ventilators are always kept at the top portion of the wall.
- The freezer region is the topmost portion in the freeze
Radiation:
Radiation is a process of transfer of heat in the form of electromagnetic waves for which material medium is not necessary. The thermal energy which is transferred by radiation is called radiant heat or radiant heat or simply radiations
Characteristics of Radiation:
- In the process of radiation thermal energy or heat energy is transferred from one point to other in the form of electromagnetic waves.
- As radiation is due to electromagnetic waves and electromagnetic waves are capable of passing through a vacuum, there is no necessity of material medium for radiation.
- Due to the electromagnetic nature of radiation has the same properties as that of light, such as rectilinear propagation, reflection, refraction, interference etc.
- The velocity of radiant energy in air or vacuum is the same as that of light in vacuum i.e 3 × 108 m/s. Due to this high-speed radiation is the most rapid process of heat transfer.
- When radiant heat is incident on a matter, it is partly absorbed and converted into heat.
- Radiations have a wavelength greater than that of red colour and thus radiation form infrared region of the electromagnetic spectrum.
Diathermanous Substances:
The substances which can transmit the radiant heat incident upon their surfaces are called diathermanous substances. e.g. glass, quartz, gases
Adiathermanous (Athermanous) Substances:
The substances which cannot transmit the radiant heat incident upon their surfaces are called adiathermanous (athermanous) substances. e.g. wood, iron copper etc.
Perfectly Black Body:
A body which absorbs all the radiant heat incident upon it is called a perfectly black body.
No body exists in nature, which can be called a perfectly black body. For practical purposes, lamp black which absorbs nearly 98 % of the heat incident upon it is considered as a perfect black body.
Characteristics of Perfectly Black Body:
- A perfectly black body which absorbs all the radiant heat incident upon.
- For a perfectly black body the coefficient of absorption is equal to 1.
- The blackness of such a body is due to the fact that it does not reflect or transmit any part of heat incident upon it. Thus the coefficient of reflection and coefficient of transmission are zero.
Applications of Radiations:
- Black bodies absorb and emit radiant energy better than bodies of lighter colours. We wear white or light coloured clothes in summer so that they absorb the least heat from the sun. However, during winter, we use dark coloured clothes which absorb heat from the sun and keep our body warm.
- The bottoms of the utensils for cooking food are blackened so that they absorb maximum heat from the fire and give it to the vegetables to be cooked.
- A Dewar flask or thermos bottle is a device to minimise heat transfer between the contents of the bottle and outside. It consists of a double-walled glass vessel with the inner and outer walls coated with silver. Radiation from the inner wall is reflected back into the contents of the bottle. The outer wall similarly reflects back any incoming radiation. The space between the walls is evacuated to reduce conduction and convection losses and the flask is supported on an insulator like a cork. The device is, therefore, useful for preventing hot contents (like milk) from getting cold, or alternatively to store cold contents (like ice).

Newton’s Law of Cooling:
The rate of loss of heat by a body is directly proportional to its excess temperature over that of the surroundings provided that this excess is small.
Explanation:
Let θ and θo, be the temperature of a body and its surroundings respectively. Let dQ / dt be the rate of loss of heat. So from Newton’s Law of Cooling,

where k is a constant.
Thus Newton’s law of cooling states that the rate of loss of heat by cooling body is directly proportional to its excess of temperature over the surrounding, provided this excess is very small.
The alternate statement of the law is that the rate of fall of temperature of a cooling body is directly proportional to its excess of temperature over the surrounding, provided this excess is very small.
dθ/dt ∝ (θ – θo)

Limitations of Newton’s Law of Cooling:
- This law is applicable when the excess temperature of a body over the surroundings is very small (about 40 °C)
- When the body is cooling the temperature of the surrounding is assumed to be constant. which is not true.
- The law is applicable for higher temperature using forced convection.
Verification of Newton’s Law of cooling:
Newton’s law of cooling can be verified with the help of the experimental set-up shown in the figure. The set-up consists of a double-walled vessel (V) containing water in between the two walls. A copper calorimeter (C) containing hot water is placed inside the double-walled vessel. Two thermometers through the corks are used to note the temperaturesT2 of water in calorimeter and T1 of hot water in between the double walls respectively.

The temperature of hot water in the calorimeter is noted after equal intervals of time. A graph is plotted between loge (T2–T1) and time (t). The nature of the graph is observed to be a straight line having a negative slope as shown in the figure.
