Science > Physics > Cathode Rays and X- Rays > X-Rays
Discovery of X- Rays:
If a discharge tube is highly evacuated so that the pressure in the tube is of the order of 0.01 mm of mercury, certain rays are emitted by the cathode in the form of bluish streamers. These rays are called as cathode rays.
In 1895, a German physicist, Rontgen accidentally discovered x-rays. He was performing experiments with the discharge tube. The room was dark and the discharge tube was covered with black cloth. There was a fluorescent screen in the darkroom. Rontgen observed that every time the discharge tube was switched on, the fluorescent screen began to glow. He placed an opaque object between the discharge tube and the fluorescent screen, a shadow of the object was seen on the screen. From these observations, Rontgen concluded that some invisible and highly penetrating rays were being emitted from the walls of the discharge tube on which cathode rays were incident. Rontgen was unable to decide the type and origin of the wave, hence he called these rays as x-rays.
In 1906, it was experimentally proved that the x-rays consist of electromagnetic waves of very short wavelengths. X-rays are also called Rontgen rays.
Production of X-Rays:
If a discharge tube is highly evacuated so that the pressure in the tube is of the order of 0.01 mm of mercury, certain rays are emitted by the cathode in the form of bluish streamers. These rays are called cathode rays. When cathode rays are suddenly stopped by a hard object, x-rays are produced.
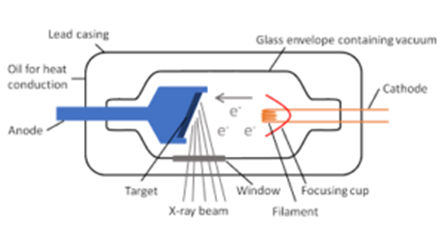
The cathode consists of a tungsten filament, which is heated by a low voltage battery. The hot filament emits electrons. These electrons are accelerated towards the target, which acts as a fixed anode which is kept at a high positive potential with respect to the filament. The target consists of a tungsten disc mounted on a copper tube, which acts as the anode.
The potential difference between the anode and the cathode can be changed from 100 V to one million volts. The cathode is surrounded by a molybdenum cylinder called ‘wehnelt cylinder’ which is maintained at a negative potential with respect to the filament. The function of the cylinder is to focus the electrons on the target. When these high energy electrons strike the target, x-rays are produced and they pass through a small part of the tube. The remaining part of the tube is covered by thick lead sheets. Only about 1% of the energy of the electrons is converted into x-rays. The remaining energy is absorbed by the target, which gets heated tremendously. Water is circulated through the copper tube to remove the heat produced.
The intensity of the x-rays depends upon the temperature of the filament. The higher the temperature, the higher is the intensity of the x-rays produced. Their penetrating power increases if the P.D. between the target and cathode is increased.
Properties of X–Rays:
- They can penetrate light materials like wood, paper, flesh, etc. which are opaque to ordinary visible light.
- The blackening on photographic plates when they are incident on them.
- They are electromagnetic waves of very short wavelength ranging from 1 to 100-angstrom unit and travel in straight lines with a velocity of light.
- Their wavelengths are shorter than those of ultraviolet rays and typically longer than those of gamma rays.
- They produce ionization in a gas through which they pass.
- When They are incident on a fluorescent substance like zinc sulphide, the substance is excited to fluorescence.
- When they are incident on a substance, electrons are emitted.
- They are not deflected by an electric and magnetic fields.
- They cannot penetrate through heavy metals and bones and cast the shadow of these objects when placed in their path.
- They cannot be reflected by ordinary mirrors or refracted by lenses and prism. They exhibit diffraction by crystals.
Uses of X–Rays:
- In the medical profession, X – rays photography called radiographs, are used to detect fractures of bones, tuberculosis of lungs, foreign matter like bullets, the formation of bones and stones in the body.
- They are used in treating cancer when surgery is not permissible. Cancer tissue is exposed to X – rays for a few minutes every day until it is completely destroyed.
- In industry, they are used to detect faults, cracks, flaws and gas bubbles in finished metal products. They are used to test weldings, castings, moulds, uniformity of an insulating substance.
- they are used for the detection of explosives and contraband goods in sealed parcels.
- They are used in scientific research such as inspecting the structure of crystals, arrangement of atoms and molecule in complex substances.