Science > Physics > Nuclear Physics > Natural Radioactivity
Radioactivity was discovered by French physicist Antoine Becquerel in 1896. He found that certain compounds of uranium emitted invisible radiations which affected photographic plates. It is also found that Thorium and its compounds also show these properties. Madame Curie and Piere Curie discovered two elements namely ‘Radium’ and ‘Polonium’ and found that they also exhibit these properties. Radioactivity can be studied under two headings natural radioactivity and artificial radioactivity. In this article, we shall study the basics of natural radioactivity.
The phenomenon of spontaneous and continuous and uncontrollable disintegration of an unstable nucleus accompanied by the emission of active radiations is called natural radioactivity. The substance which exhibits radioactivity is called a radioactive substance. e.g. Uranium, thorium, radium, etc.
Characteristics of Natural Radioactivity:
- These characteristics are also called as Rutherford-Soddy’s radioactive disintegration theory.
- Natural radioactivity is a purely nuclear phenomenon. The nucleus of a radioactive substance is unstable and such an unstable nucleus undergoes spontaneous breakdown (disintegration). The process continues until a stable nucleus is obtained.
- As Natural radioactivity is the nuclear phenomenon it is unaffected by chemical combination. I.e. the element will exhibit radioactivity in free as well as the combined state.
- Natural radioactivity is a spontaneous process. It is independent of external factors like temperature, pressure, and state of the existence of substance or catalytic action. Hence the process of radioactive disintegration is uncontrollable using these factors.
- The nucleus of the radioactive element emits alpha, beta particles, and gamma radiations and gets converted into the nucleus of another element.
- The element undergoing disintegration is called a parent element and a new element formed is called a daughter element. The daughter element has different chemical and physical properties as compared with that of its parent element.
- During disintegration, besides emission of alpha and beta particles and gamma radiation, a large amount of energy is liberated in the form of gamma rays. When gamma rays are given out no new element is formed.
- The time taken by a radioactive substance to disintegrate half of its initial quantity is called a half-life period. A half-life period is a characteristic property of every radio element.
- When radioactive substance emits one alpha particle mass number of daughter element reduces by 4 units and the atomic number reduces by 2 units. When a radioactive substance emits one beta particle, the atomic number of daughter element increases by one unit but the mass number remains unchanged.
- The rate of disintegration at any instant is directly proportional to the radioactive nuclei present at that instant.
- Thus the rate of disintegration depends on nature and the original amount of the radioactive substance.
- No radioactive substance emits both alpha and beta particles simultaneously. Gamma rays are emitted along with both alpha and beta particles.
Rutherford’s Experiment to Study Natural Radioactivity:
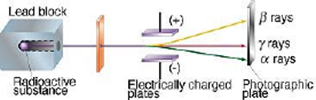
Rutherford analyzed the radiations of radioactive substances. He reported three types of radiations depending upon the effect of the magnetic or electric field upon them. These are alpha and beta particles and gamma rays.
The apparatus consists of an evacuated metal chamber with a photographic plate at the top. A small quantity of the radioactive substance is placed in a hole of the lead block. A strong electric field is applied between the plates.
When there is no electrical field the radioactive emissions move in a straight line but when the electrical field is applied the emission gets split into three distinct points on the photographic plate.
Observations and Conclusion:
- The rays which get deviated towards the negative plate are positively charged and are called as alpha rays. The deflection of alpha particles is slightly less.
- The rays which get deviated towards the positive plate are negatively charged and are called as beta rays. The deflection of beta particles is more.
- The rays which do not get deviated and move straight are not charged and are called gamma rays.
- By applying a strong uniform magnetic field at right angles to the diagram the same effect can be observed.
Effect of Magnetic Field:
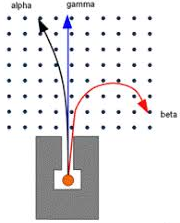
Characteristics of α – rays:
- These are positively charged particles. So α-rays are called α – particles rather than α -rays.
- Actually, these particles are helium nuclei (2He4) having 4 unit mass and 2 units of a positive charge.
- They are deflected towards the negative plate of the electric field and the magnetic field.
- The small deflection in the electric or the magnetic field indicates that they are comparatively heavier particles.
- They have greater ionizing power (100 times that of beta particles and 10000 times that of gamma rays). Their tracks in cloud chamber are continuous.
- They have the least penetration power ( 1/100 that of beta particles and 1/10000 that of gamma rays). They can be stopped by 0.1 mm thick aluminium.
- They are scattered when passing through the foils of gold and mica. This property was used by Rutherford to propose the planetary model of an atom.
- They produce fluorescence in substances like zinc sulphide and barium platinocyanide. Using scintillations on the fluorescent screen the number of alpha particles can be counted.
- They can affect a photographic plate.
- They travel in a straight line.
- They produce heating when stopped.
- They have a velocity which is about 1/10th that of light. The velocities of alpha particles emitted by different radioactive materials are different but for the same element, the velocity is the same.
- Their range varies from substance to substance. In the air, it is 2.7 cm for uranium and 8.7 cm from thorium. It also depends on the pressure of the medium.
- When radioactive substance emits one µ -particle, the mass number of the daughter element reduces by 4 units and atomic number by 2 units.
- They produce incurable burns on the human body.
Uses of Alpha Particles:
- Alpha particles are most commonly used in smoke alarms. These alarms contain a tiny amount of decaying Americium between two sheets of metal. The decaying Americium emits alpha radiation. A small electric current is then passed through one of the sheets and into the second one. When the field of alpha radiation is blocked by smoke, the alarm goes off. This alpha radiation is not harmful because it is much localized and any radiation that might escape would be stopped quickly in the air and would be extremely difficult to get into the human body.
- Due to the high velocity of emission alpha particles are used for bombarding the nuclei in the transmutation of one element into other.
Characteristics of β-rays :
- β – Rays are negatively charged particles. So they are called β – particles rather than β – rays.
- β – particles are nothing but high-velocity electrons (-1e0) having a unit negative charge (1.6 x 10-19 C) and negligible mass. These are not orbital electrons. They are electrons emitted by an atom.
- These rays are deflected towards the +ve plate of the electric field or the magnetic field. Their deflection is larger than that of alpha particles.
- The range of beta particles for a particular radioactive substance is not definite.
- They have less ionizing power as compared with that of α- rays. It is 1/100 that of alpha particles and 100 times as that of gamma rays. As beta particles cannot produce ionization continuously, their tracks in cloud not appear to be continuous.
- They have greater penetration power than that of α – rays. It is 100 times that of alpha particles and 1/100 times that of gamma rays. It can pass through a 1 mm thick sheet of aluminium.
- They produce fluorescence in substances like calcium tungstate, zinc sulphide and barium platinocyanide.
- They affect a photographic plate to a much higher extent than the α – particles.
- They do not travel in a straight line.
- They have a greater velocity than that of the α- rays very close to that of light. There is enough variation in the velocities of beta particles emitted by the same radioactive material. Hence variation in the extent of deflection in the electric field and the magnetic field is observed.
- Since the velocity of beta particles is comparable with that of light, their mass increases with the increase in their velocity. The new mass is given by the expression.

Where mO = rest mass of electron
v = velocity of beta particle
c = velocity of light
- When a radioactive substance emits one β -particle, the atomic number of daughter element increases by one unit but the mass number remains unchanged.
Characteristics of γ – rays.
- gamma rays are non-material waves.
- They are electromagnetic radiations.
- They are chargeless, hence remain undeflected due to the electric or the magnetic field.
- They have very low ionizing power.
- They have high penetration power. They can pass through a 30 cm thick iron block.
- They have more effect on a photographic plate than beta particles..
- They travel in a straight line.
- They are diffracted by crystals in the same way as X-rays.
- They have a velocity equal to that of the light.
- When radioactive substance emits gamma rays there is no change in the atomic number and the mass number.
- They are absorbed by substances and give rise to the phenomenon of pair production. They strike the nucleus of some atom, where they lose their existence and an electron and a positron are formed.

It is to be noted that gamma rays are similar to X-rays but their sources of origin are different. X-rays are produced by the transition of electrons in one energy level to another energy level. Thus it is atomic property. While gamma rays are produced due to nuclear activity. Thus gamma rays are nuclear property.
Soddy’s Group Displacement Laws:
- Whenever the parent element emits an α- particle, the daughter element produced has the atomic number less by 2 units and the mass number less by 4 units, so the daughter element occupies 2 positions to the left with respect to its parent element in the periodic table.
- Whenever the parent element emits one β – particle, the daughter element produced has the atomic number greater by 1 unit but the mass number remains the same. So the daughter element occupies one position to the right with respect to its parent element in the periodic table.
- Whenever the parent element emits one γ – ray, the daughter element produced has the same atomic number and the same atomic mass. So the daughter element occupies the same as its parent element in the periodic table.
Example:

Next Topic: The Concept of radioactive Decay
One reply on “Natural Radioactivity”
Oh,,, my God!!! What a resource site is this…
I enjoyed and understand the write up and explanation..