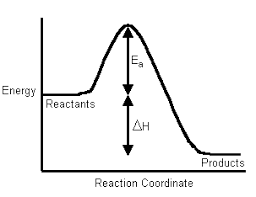
Science > Chemistry > Chemical Thermodynamics and Energetics > Heat of Reaction Of Different Processes In this article, we shall study change in enthalpy for different chemical processes. Enthalpy of Formation (ΔfH° or ΔformationH°): The change in enthalpy of a chemical reaction at a given temperature and pressure, when one mole of the substance is formed […]
Categories
Enthalpy Of Different Processes
- Post author By Hemant More
- Post date January 20, 2020
- No Comments on Enthalpy Of Different Processes
- Tags Adiabatic process, Bond energy, Bond enthalpy, Chemistry, Constant pressure process, Constant temperature process, Constant volume process, Crystal lattice energy, Cyclic process, Endothermic reaction, Enthalpy of atomization, Enthalpy of combustion, Enthalpy of condensation, Enthalpy of dissociation, Enthalpy of formation, Enthalpy of freezing, Enthalpy of fusion, Enthalpy of hydration, Enthalpy of ionization, Enthalpy of neutralization, Enthalpy of reaction, Enthalpy of solution, Enthalpy of sublimation, Enthalpy of vapourization, Exothermic reaction, Free expansion, Fusion, Heat of reaction, Irreversible process, Isobaric process, Isochoric process, Isothermal process, Pressure volume work, Process, Reversible, Reversible process, Sign convention, State of a substance, Sublimation, Thermochemical equation, vapourization, work done in cyclic process