Science > Chemistry > Chemical Thermodynamics and Energetics > Heat of Reaction
The branch of chemistry which deals with the quantitative study of thermal or heat changes in various chemical reactions is known as thermochemistry. In this article, We shall discuss very important concept of chemistry i.e. heat of reaction.
Thermochemical Equation:
An equation which indicates the heat changes in a chemical reaction at a certain temperature and pressure with an indication of states of reactants and products is called a thermochemical equation
Example:
C(s) + O2(g) → CO2(g) , ΔH = -395.39 kJ
This thermochemical equation indicates that when one mole of solid carbon reacts with one mole of gaseous oxygen at constant pressure one mole of gaseous carbon dioxide is obtained. During this reaction, 395.39 kJ of heat is evolved.
Guidelines for Writing Thermochemical Equation:
- The equation must be automatically balanced like a chemical equation.
- The value of ΔH must be indicated. ΔH is negative for exothermic reaction and positive for an endothermic reaction.
- The physical state of each reactant and each product must be indicated. Symbols such as (s) for solid-state, (l) for the liquid state, (g) for gaseous state and (aq.) for an aqueous solution should be indicated.
- The thermochemical equation can be reversed. During the reversal of the sign of ΔH must be changed.
- The temperature of the reaction can be written as a suffix to ΔH
Example:
C(s) + O2(g) → CO2(g) , ΔH = -395.39 kJ
This thermochemical equation indicates that when one mole of solid carbon reacts with one mole of gaseous oxygen at constant pressure one mole of gaseous carbon dioxide is obtained. During this reaction, 395.39 kJ of heat is evolved.
The necessity of Mentioning of State of a Substance:
It is important to mention the physical state of substances in the thermochemical equation because the change of physical state is also accompanied by the enthalpy change.
Example: Consider the following thermochemical equations
H2(g) + 1/2O2(g) → H2O(l) , ΔH = – 286 kJ
Thus, when 1 mole of hydrogen gas reacts with half a mole of oxygen gas to form one mole of liquid water, 286 kJ of heat is produced.
H2(g) + 1/2O2(g) → H2O(g) , ΔH = – 249 kJ
Thus, when 1 mole of hydrogen gas reacts with half a mole of oxygen gas to form one mole of water vapours, 249 kJ of heat is produced. Hence, whenever there is a state change, there is a change in enthalpy of reaction. Hence in thermochemical reaction, the state of each substance involved should be mentioned.
Heat of Reaction OR Enthalpy of Chemical Reaction:
The difference between the sum of enthalpies of products and the sum of enthalpies of reactants at a given temperature is called as the heat of reaction. It is denoted by ΔH or ΔU
Explanation:
Consider a general chemical reaction
A + B → C + D.
Let HA , HB, HC, and HD be the enthalpies of A, B, C and D respectively then the heat of reaction is given by
ΔH = (HC + HD) – (HA + HB)
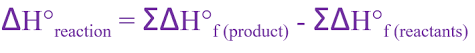
The heat of reaction can be determined either at constant pressure or at constant volume.
Heat of Reaction at Constant Pressure:
The difference between the sum of enthalpies of products and the sum of enthalpies of reactants at a given temperature and constant pressure is called the heat of reaction at the constant pressure at a given temperature.
It is denoted by ΔH. Normally heat of reaction at constant pressure is specified at 298 K and 1 atm. Pressure. This heat of reaction is called the standard heat of reaction.
Thus at constant pressure heat of reaction is given by
ΔH = ∑ ΔHProducts – ∑ ΔHReactants
ΔH is negative for exothermic reaction and positive for an endothermic reaction.
Heat of Reaction at Constant Volume:
The difference between the sum of internal energies of products and the sum of internal energies of reactants at a given temperature and constant volume is called the heat of reaction at the constant volume at a given temperature. It is denoted by ΔE.
Thus at constant pressure heat of reaction is given by
ΔU = ∑ ΔUProducts – ∑ ΔUReactants
Factors Affecting Heat of Reaction:
- Physical states of substances involved.
- The amount of substance involved.
- Way of carrying out the reaction i.e. in a case of gaseous reactions heat of reaction depends on whether the reaction is carried out at constant pressure or at constant volume.
- The pressure of reactants and products.
- The temperature (as explained by Kirchhoff’s equations)
Different Types of Chemical Reactions on the Basis of Change in the Enthalpy:
On the basis of change in the enthalpy chemical reactions are classified into exothermic reactions and endothermic reactions
Exothermic Reactions:
The chemical reactions which involve the evolution of heat are called as exothermic reactions.
Example:
C(s)+ O2(g) → CO2(g) , ΔH = -395.39 kJ
In this case, the enthalpy of products is less than the enthalpy of reactants. For such reactions, the change in enthalpy is always negative.
Characteristics of Exothermic Reactions:
- The chemical reactions which involve the evolution of heat are called exothermic reactions.
- For such reactions, the change in enthalpy is always negative.
- In this case, the enthalpy of products is less than the enthalpy of reactants
- Products are more stable than the reactants.
Endothermic Reactions:
The chemical reactions which involve absorption of heat are called endothermic reactions.
Example :
2C(s)+ H2(g) → C2H2(g) , ΔH = + 225.94kJ
In this case, the enthalpy of products is more than the enthalpy of reactants. For such reactions, the change in enthalpy is always positive.
Characteristics of Endothermic Reactions:
- The chemical reactions which involve absorption of heat are called endothermic reactions.
- For such reactions, the change in enthalpy is always positive.
- In this case, the enthalpy of products is more than the enthalpy of reactants.
- Products are less stable than the reactants.
Previous Topic: Numerical Problems on Enthalpy and Internal Energy Changes
Next Topic: Change in Enthalpy in Different Processes