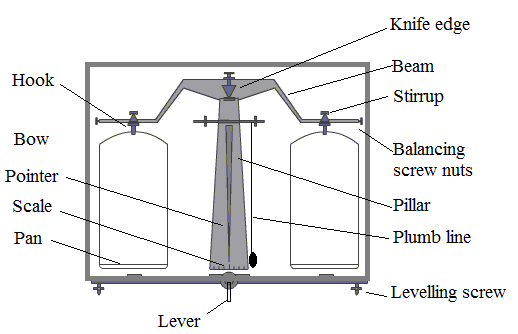
Science > Physics > Units and Measurements > Measurement of Density In this article, we shall study the measurement of the density of a substance. Eureka! Eureka! Archimedes was a Greek scientist. He lives in Syracuse nearly 200 years ago. The King of the land wanted to wear a Golden Crown. He gave some gold […]
Measurement of Density