Science > Chemistry > Colloids > Associated Colloids (Micelles)
In thisarticle, we shall study bout associated colloids :micelles).
Multimolecular Colloids:
Multimolecular colloids are those systems in which the dispersed phase particles are aggregates of many atoms or molecules. The particles in this colloidal solutions are held together by van der Wall’s forces. e.g. gold sol particles are an aggregation of many gold atoms. Other examples are silver sol and sulphur sol.
Macromolecular Colloids:
Macromolecular colloids are those systems in which the dispersed phase particles are a single macromolecule. They are lyophilic in character. e.g. sol of starch in water, Aqueous (Water) solution of proteins, enzymes.
Associated Colloids or Micelles
Colloids which behave as normal electrolytes at low concentrations, but exhibit colloidal properties at higher concentrations due to the formation of aggregated particles called associated colloids. The aggregated particles thus formed are called micelles.
The associated colloids are usually formed by surfactants (surface active agents) like soaps and synthetic detergents. The molecules of soaps and detergents are smaller than the colloidal particles. When dissolved in water soap and detergent molecules act as an electrolyte but if their concentration is increased then their molecules aggregate to form colloidal size particles called micelles. The formation of micelles takes place only above a certain concentration, this concentration is known as critical micellization concentration (CMC).
The Process of Formation of Micelles:
The formation of a micelle can be understood by taking the example of a soap solution. In general, soap can be represented as RCOONa, where R represents a long chain alkyl group. When dissolved in water, soap ionizes to give RCOO– and Na+ ions. The most commonly used washing soap is sodium stearate, C17H35COONa.
Clothes become dirty due to the deposition of dust and oily or greasy substances. Water is not capable of wetting oily or greasy substances. However, the hydrocarbon residue R of the soap anion (RCOO–) can wet the oily or greasy substances. Soaps and detergents have two parts, the hydrophobic part and the hydrophilic part.
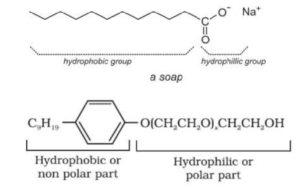
Hydrophobic or water repelling non-polar part (usually a long hydrocarbon chain) is soluble in oil and greases but insoluble in water. Hydrophilic or water attracting polar parts such as carboxylic group or sulphonate or sulphate is soluble in water and insoluble in oil and greases.
Molecules of soap and detergent form micelles in water. The hydrophobic part of soap dissolves in oil and grease while hydrophilic part of soap remains as free in soap solution. When the cloth is rubbed with hand or stirred mechanically, the big molecules of oil and soap break into small emulsified oil droplets. These oil droplets repel each other due to the presence of anions of the hydrophilic part and do not precipitate. Thus they remain suspended in the soap solution without getting back on the cloth. These suspended oil and grease particles are then washed away by a stream of water.
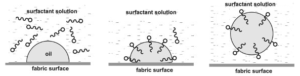
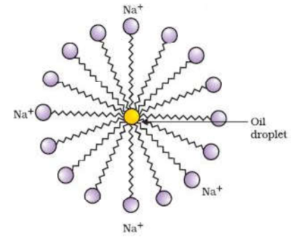
In micelle formation, the long hydrocarbon chain which is insoluble in water is directed towards the centre while the soluble polar head is on the surface in contact with water.
The cleaning action of detergents such as sodium lauryl sulphate, CH3 (CH2)11SO4Na+ or sulphonates of long-chain hydrocarbons is similar to that of soaps. In case of detergents, the polar groups are sulphate (–SO4) or sulphonate (-SO3) groups.
Kraft’s Temperature:
The formation of micelles takes place only above a particular temperature is called the kraft’s temperature.
More Examples of Micelle System:
Sodium lauryl sulphate:

Sodium oleate:

Cetyltrimethyl ammonium bromide:

It forms micelle with a cationic terminal.
p-Dodecyl benzene sulphonate:

Surfactants:
Surfactants are compounds that lower the surface tension. They are preferentially adsorbed at the interfaces between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants.
Cationic Surfactants:
The cationic surfactants are quaternary ammonium compounds with aryl or alkyl substituent groups, one of which is often a long hydrophobic carbon chain. Cationic surfactants are positively charged, and therefore are not as effective as detergents in cleansing systems. They can ideally be used as fabric softener.
Cetyl pyridinium chloride:

Cetyltrimethyl ammonium chloride:

Octadecyl ammonium chloride

Anionic Surfactants:
Anionic surfactant gives anion. Anionic surfactants are positively charged and are widely used. Anionic surfactants possess a negative charge on their hydrophilic end. Generally, they make a lot of foam when agitated. They are free-flowing powdery when dry, not sticky like other surfactants.
Sodium Palmitate:

Sodium oleate:

Salts of sulphonic acid
Non-ionogenic or Non-ionic Surfactants:
Nonionic surfactants have no charge on their hydrophilic end, hence they are used as superior oily soil emulsifiers. They do not ionise or dissociate in an aqueous medium. Because of their lower foam profile and strong emulsifying potential, these surfactants are the preferred choice when formulating extraction cleaners and pre-sprays. Nonionics are thick liquids or syrups that are sticky. Nonionic surfactants include: ethoxylates, alkoxylates and cocamide
Characteristics of Micelles
- The formation of micelles takes place only above a certain concentration, this concentration is known as critical micellization concentration (CMC). As the concentration of a surfactant increases, adsorption takes place at the surface until it is fully overlaid, which corresponds to the minimum value of the surface tension.
- An increase in temperature usually increases CMC.
- Greater the chain length of the hydrocarbon chain smaller is the CMC.
- An increase in the hydrophobic part of the surfactant increases CMC.
- The addition of simple electrolyte in ionic micelles decreases their CMC.
- The formation of micelles takes place only above a particular temperature is called the Kraft’s temperature. Below Kraft’s temperature the solubility of surfactant not enough to form micelles.
- Kraft’s temperature increases with the increase in the number of carbon atoms.

One reply on “Associated Colloids (Micelles)”
It is more informative