Science > Chemistry > Colloids > Preparation of Colloids
In this article, we shall study different methods of preparation of colloids.
Preparation of Lyophilic Sols:
For preparing lyophilic sol, the dispersed phase is directly added to dispersion medium in cold or by warming.
Colloidal solutions of starch, glue, gelatin, etc. in water can be prepared by this method. Solutions of colloidal electrolytes such as soaps and dyestuffs can also be prepared by this method.
Preparation of Lyophobic Sols:
For preparing lyophobic sol, the substance in bulk is broken down into particles of colloidal dimensions (Dispersion) or aggregating smaller particles into particles of colloidal dimensions (condensation). To improve the stability of sol certain substances are added to the sol, the substances added are called stabilizers.
Dispersion methods:
In the dispersion method particle of larger size are broken down to the colloidal size in the dispersion medium. Starting with the material in massive form, a colloidal solution is prepared by using suitable devices to disintegrate it into particles of colloidal size. Normally this is carried out by physical methods.
Mechanical dispersion method :
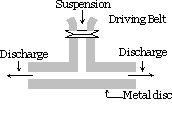
The substance which is to be dispersed is finely ground. It is then mixed with the dispersion medium, protective materials or stabilizer is also added when a coarse suspension is obtained. This suspension is then passed through a colloid mill. A colloid mill consists of two heavy metal discs placed one above the other separated by a very small gap from each other. They are rotated in the opposite directions at a very high speed of about 7000 r.p.m. The sol results due to the large shearing effect. Protective material used prevents particles from coagulation.
Using this method sols of indigo, sulphur, toothpaste, printer ink, paints, ointments etc. are prepared.
Electrical Dispersion or Bredig’s Arc Method:
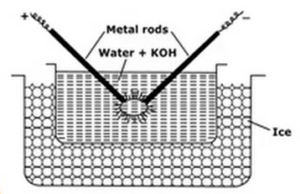
This method used to prepared metal sols like platinum, silver, gold, copper in water. A dispersion medium (conductivity water) and a trace of sodium hydroxide (the stabilising agent) is taken in porcelain or glass (non conducting) vessel. The vessel containing dispersion medium is surrounded by a freezing mixture. Metal to be dispersed is dipped in the vessel in the form of electrodes. Electrodes are connected to the high voltage source. The ends of electrodes in the dispersion medium are very near to each other. A very high voltage is applied and then an electrical arc is struck between the tips of electrodes. This creates large heat due to which metal rods melt, evaporate and suddenly cooled due to freezing mixture gives rise to the colloidal solution of the metal.
Functions of the freezing mixtures are
- Freezing mixture helps in condensation of metal vapours forming the colloids
- It prevents vapourisation of water.
- It prevents coagulation of colloids, by keeping sol cold.
Peptization or Chemical Dispersion:
Redispersion of freshly prepared precipitate into the sol by adding an electrolyte containing common ion is called as peptization. An electrolyte used for peptization is called as the peptizing agent. Peptization is a reverse process of coagulation. The peptization action is due to the preferential adsorption of one of the ions of the electrolyte on the precipitate.
Example – 1:
Freshly prepared Fe(OH)3 precipitate when treated with dilute solution of FeCl3, reddish brown ferric hydroxide sol is formed (Fe3+ being common ion). In this case, FeCl3 is the peptizing agent.
Fe (OH)3 + FeCl3 → [Fe(OH)3] Fe3+
Example – 2:
Fresh Silver chloride precipitate when treated with a small amount of dilute HCl, a silver chloride sol is formed.
Example – 3:
Cadmium sulphate can be peptized with the help of hydrogen sulphate.
Ultrasonic Dispersion:
Ultrasound is a very effective processing method in the generation and application of colloidal size particles. High-intensity ultrasonic waves are used for this purpose. During sonicating of liquids the ultrasonic waves that propagate through dispersion medium result in alternating high-pressure (compression) and low-pressure (rarefaction) cycles. This mechanical stress causes Ultrasonic cavitation in liquids. It creates high-speed liquid jets of up to 1000km/h. Such jets press liquid at high pressure between the particles and separate them from each other. Smaller particles are accelerated with the liquid jets and collide at high speeds.
Various substances like oils, mercury, sulphur, sulphides and oxides of metal can be dispersed in a colloidal state by this method.
Condensation methods:
These methods involve chemical reactions. In these methods factors like temperature, pressure, concentrations. etc. are properly maintained. The unwanted ions present in the sol are removed by dialysis , as these ions may eventually coagulate the sol.
Chemical Methods:
Oxidation Method:
Preparation of Colloidal Sulphur:
When H2S in water (aqueous solution) is exposed to air, it slowly gets oxidised to sulphur. The sulphur so formed remains in water in the colloidal state and the solution so formed remains in water in the colloidal state and the solution has a slightly milkish appearance.
H2S + O2 → H2O + 2S (colloidal)
A sol of sulphur can also be prepared when H2S gas is bubbled through an aqueous solution of SO2.
H2S + SO2 → 2 H2O + 3S (colloidal)
Reduction Method:
Preparation of Gold Sol:
A number of metals like silver, gold, platinum, mercury lead can be obtained in the colloidal state by the reduction of their salt solutions (dilute) using suitable reducing agents like hydrogen sulphide, formaldehyde, stannous chloride, tannic acid etc.
Gold sol can be obtained when AuCl3(dil) solution is treated with stannous chloride.
2 AuCl3 + 3 SnCl2 → 3 SnCl4 + 2 Au (colloidal)
Similarly, silver, platinum mercury sols are prepared.
AgNO3 + Tannic acid → Ag sol
AuCl3 + Tannic acid → Au sol
Hydrolysis Method:
Preparation of Ferric Hydroxide Sol:
A colloidal solution of ferric hydroxide is obtained by boiling a dilute solution of ferric chloride.
FeCl3 + 3H2O → Fe(OH)3 + 3 HCl
Preparation of Silicic Acid Sol:
By hydrolysis of a dilute solution of sodium silicate with a hydrochloric acid, the colloidal solution of silicic acid is obtained.
Double Decomposition Method:
Preparation of Arsenious Sulphide Sol:
Arsenious sulphide, As2S3 is a lyophobic colloid. It is obtained by the hydrolysis of arsenious oxide (AS203) with boiling distilled water, followed by passing H2S gas through solution obtained. In the colloidal solution of arsenious sulphide, each particle is surrounded by HS- ions, produced by the dissociation of H2S. This sulphide ion layer is further surrounded by the counter ion layer of H+ ions.
As2O3 + 3 H2O → 2As(OH)3 (boiling)
2 As(OH)3 + 3H2S → As2S3 + 6H2O
(light yellow sol)
By Exchange Solvent Method:
There are a number of substances whose colloidal solutions can be prepared by taking a solution of the substance in one solvent and pouring it into another solvent in which the substance is relatively less soluble.
Preparation of Sulphur or Phosphorous Sol:
If a solution of sulphur or phosphorus prepared in alcohol is poured into water, a colloidal solution of sulphur or phosphorus is obtained due to the low solubility of sulphur or phosphorus in water.
By change of physical state:
A colloidal solution of certain elements such as mercury and sulphur are obtained by passing their vapours through cold water containing a stabilizer ( an ammonium salt or a citrate).
Excessive Cooling Method:
A colloidal solution of ice in an organic solvent like ether or chloroform can be prepared by freezing a solution of water in the solvent. The molecules of water which can no longer be held in solution, separately combine to form particles of colloidal size.
Purification of Colloidal Solution
Dialysis:
The process of separating the particles of colloid from those of crystalloid, by means of diffusion through a suitable membrane (animal membrane or parchment paper) is called dialysis. The apparatus used for the performing dialysis is called dialyser.
Principle:
The colloidal particles can not pass through a parchment or cellophane membrane while the ions of the electrolyte (crystalloids) can pass through it.
Process:
A bag made up of suitable semipermeable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flown. The molecules and ions of crystalloids diffuse through the membrane into the water and are washed away. Thus the sol in the bag is purified.
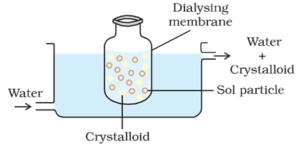
Dialysis can be used for removing HCl from the ferric hydroxide sol.
Electrodialysis:
The ordinary process of dialysis is slow. (ii) To increase the process of purification, the dialysis is carried out by applying an electric field. This process is called electrodialysis. The ions present in the colloidal solution migrate towards oppositely charged electrodes.

The important application of electrodialysis process in the artificial kidney machine used for the purification of the blood of the patients whose kidneys have failed to work.
Ultrafiltration:
The pores of ordinary filter paper are large, hence colloidal particles pass through them easily. If the pores of the ordinary filter paper are made smaller by soaking the filter paper in a solution of gelatin of colloidion (it is a mixture of 4% nitro-cellulose in alcohol and ether) and subsequently hardened by soaking in formaldehyde
The treated filter paper may retain colloidal particles and allow the true solution particles to escape. Such filter paper is known as ultrafilter and the process of separating colloids by using ultrafilters is known as ultrafiltration.
The colloidal particles left on ultrafilter paper are then washed with a fresh dispersion medium to get a pure colloidal solution.
Ultracentrifugation:
In this method, the colloidal solution is placed in a high-speed centrifugal machine. On centrifuging, the colloidal particles settle down. The impurities remain in the centrifugate and are removed. The settled colloidal particles are mixed with the dispersion medium to form the colloidal solution again.
One reply on “Preparation of Colloids”
I really enjoyed this note , the note is very simple and clear .. thank you very much… post more on others topice,I’ve the petroleum chemistry..