Science > Chemistry > Chemical Thermodynamics and Energetics > Chemical Processes
In this article, we shall discuss what is meant by process and different types of chemical processes.
Process:
It is the path or the operation by which a system changes from one state to another. A process causes a change in the value of at least one of the state functions.
Types of Chemical Processes:
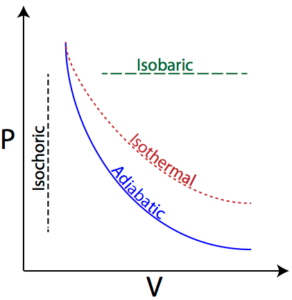
Isothermal process:
If a process is carried out at a constant temperature, the process is called an isothermal process. For isothermal process ΔT = 0, ΔU = 0. Internal energy (U) of a system remains constant during isothermal process provided there is no change of phase. e.g. Fusion of ice.
Characteristics of Isothermal Chemical Processes:
- If a process is carried out at a constant temperature, the process is called an isothermal process.
- The exchange of heat takes place with the surroundings. ( q ≠ 0)
- Internal energy remains constant. Δ U = 0 (provided there is no change in a phase).
- Total heat content (enthalpy) of the system varies.(Δ H ≠ 0)
- The system is not thermally isolated from the surroundings.
- Expansion occurs with the absorption of heat, while compression occurs with the evolution of heat.
- W = q
- An isothermal process can be made reversible.
Adiabatic process:
A process carried out in such a manner that the system, undergoing the change, does not exchange heat with the surroundings is called an adiabatic process. The temperature of the system changes during the adiabatic process. For adiabatic process Q = 0. e.g. Expansion of a gas in a vacuum.
Characteristics of Adiabatic Chemical Processes:
- If a process is carried out in such a manner that the system, undergoing the change, does not exchange with the surroundings is called an adiabatic process.
- The exchange of heat with the surrounding does not take place. ( q = 0)
- Internal energy varies. (ΔE ≠ 0)
- Total heat content (enthalpy) of the system remains constant.(Δ H = 0)
- The system is thermally isolated from the surroundings.
- In expansion temperature and internal energy decreases, while in compression temperature and internal energy increase.
- W = E2 – E1 = Δ E
- The adiabatic process cannot be made reversible.
Isobaric process:
If a process is carried out at constant pressure, the process is called an isobaric process. For isobaric process ΔP = 0.
Example: The reaction carried out an open vessel i.e. at constant atmospheric pressure.
Isochoric process:
If a process is carried out at constant volume, the process is called the isochoric process. For isochoric process ΔV = 0, W = 0.
Example: A gaseous reaction carried out in a tightly closed container.
Reversible process:
A thermodynamically reversible process is one which takes place infinitesimally slowly in a large number of stages so that at every stage driving force is only infinitesimally greater than the opposing force and direction of the process at any stage can be reversed by an infinitesimal increase in opposing force.
At every stage of the process, the system remains in thermodynamic equilibrium with the surroundings.
Characteristics of Reversible Chemical Processes:
- A reversible process is one whose direction can be reversed by an infinitesimal change in the conditions.
- It is a hypothetical (imaginary) reaction. It is a non-spontaneous process. It is to be arranged artificially.
- This process is infinitesimally slow
- There is an equilibrium at every stage of the process.
- The direction of the process can be reversed at any stage by an infinitesimal change in one of the state functions.
- The driving and opposing forces differ by an infinitesimally small amount.
- Maximum work can be obtained.
Irreversible process:
In an irreversible process, the system does not remain in thermodynamic equilibrium with surrounding during the process. Equilibrium is reached at the end of the process.
In an irreversible process driving and opposing forces differ by a finite amount and process proceeds in a definite direction, its direction cannot be reversed by very small changes of conditions.
An irreversible process is a natural spontaneous process like the flow of water from a higher level to a lower level.
Characteristics of Irreversible Chemical Processes:
- An irreversible process is one whose direction cannot be reversed by changing the conditions slightly.
- It is a natural and real process. It is a spontaneous process. It takes place naturally.
- This process is comparatively fast.
- Equilibrium is reached at the end of the process
- The direction of the process cannot be reversed by small changes in state functions.
- The driving forces are quite larger than opposing forces.
- Maximum work cannot be obtained.
Cyclic process:
A cyclic process is one which consists of a series of intermediate steps at the end of which the system returns to the initial state. Since in a cycle, initial and final states are the same, Thus, Δ (state function) = 0
Representation of Various Chemical Processes on PV Diagram:
Previous Topic: Introduction to Chemical Thermodynamics
Next Topic: Concept of Internal Energy
One reply on “Chemical Processes”
very good note