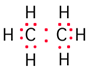
Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Formation of Covalent Bonds
In previous articles, we have seen formation of ionic bonds. In this article, we shall study the concept of the formation of a covalent bond.
Covalent Bonds or Electron Pair Bonds:
A bond established between two identical or different atoms by sharing one or more pairs of electrons is known as a covalent bond. The attractive force which comes into existence due to the mutual sharing of electrons between two atoms having similar electronegativity or having a small difference in electronegativities is called a covalent bond.
No ionic bond is possible between two atoms having similar electronegativities. Hence to explain the bonding between such atoms Lewis introduced the concept of covalent bonds.
Each atom contributes one electron to form a common pair i.e. equal contribution of electrons followed by equal sharing. If one electron pair is shared, it is known as a single covalent bond. If two electron pairs are shared, it is a double covalent bond and so on. In CI2, O2, CH4, H2O, etc. there are covalent bonds.
Examples of Covalent Bonds:
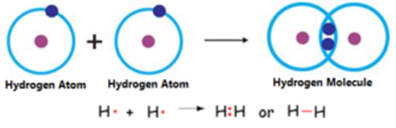
Formation of Hydrogen Molecule:
Electronic configuration of hydrogen H (Z = 1) is (1) Hydrogen has one electron in its valence shell. It tries to acquire a stable configuration (2) by sharing its one electron with another hydrogen atom.
When two hydrogen atoms approach each other, at a certain distance between the nuclei, they share their valence electrons and form a shared pair of electrons. The shared pair belongs to each atom equally. As only one pair is shared between the two hydrogen atoms, they are joined to each other by a single covalent bond.
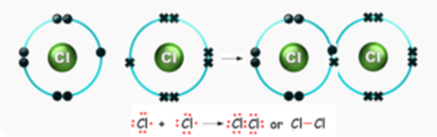
Formation of Chlorine Molecule:
Electronic configuration of chlorine Cl (Z = 17) is (2, 8, 7). Chlorine has 7 electrons in its valence shell. It can acquire a stable configuration (2, 8, 8) of the nearest inert gas (Ar) by sharing one electron with another chlorine atom.
When two chlorine atoms approach each other, at a certain distance between the nuclei, they share their valence electrons and form a shared pair of electrons. The shared pair belongs to each atom equally. As only one pair is shared between the two chlorine atoms, they are joined to each other by a single covalent bond.
Formation of Hydrogen Chloride Molecule:
Electronic configuration of hydrogen H (Z = 1) is (1) Hydrogen has one electron in its valence shell. It tries to acquire a stable configuration (2) by sharing its one electron with the chlorine atom. Electronic configuration of chlorine Cl (Z = 17) is (2, 8, 7). Chlorine has 7 electrons in its valence shell. It can acquire a stable configuration (2, 8, 8) of nearest inert gas (Ar) by sharing one electron with the hydrogen atom.
When hydrogen and chlorine atoms approach each other, at a certain distance between the nuclei, they share their valence electrons and form a shared pair of electrons. The shared pair belongs to each atom equally. As only one pair is shared between the hydrogen and chlorine atoms, they are joined to each other by a single covalent bond.

Formation of Ammonia Molecule:
Electronic configuration of nitrogen N (Z = 7) is (2, 5). Nitrogen has 5 electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by sharing one electron each with three hydrogen atom.
When three hydrogen atoms approach nitrogen atom, at a certain distance between the nuclei, it shares one valence electron each with the nitrogen atom and forms three shared pairs of electrons with three hydrogen atoms. The shared pair belongs to each atom equally. As only one pair is shared between the atoms, they are joined to each other by a single covalent bond.

Formation of Water Molecule:
Electronic configuration of oxygen O (Z = 8) is (2, 6). Oxygen has 6 electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by sharing one electron each with two hydrogen atom.
When two hydrogen atoms approach oxygen atom, at a certain distance between the nuclei, it shares one valence electron each with two hydrogen atoms and forms two shared pairs of electrons with two hydrogen atoms. The shared pair belongs to each atom equally. As only one pair is shared between the atoms, they are joined to each other by a single covalent bond.

Formation of Phosphorous Trichloride Molecule:
Electronic configuration of phosphorous P (Z = 15) is (2, 8, 5). Phosphorous has 5 electrons in its valence shell. It can acquire stable configuration (2, 8, 8) of nearest inert gas (Ar) by sharing one electron each with three chlorine atoms. Electronic configuration of chlorine Cl (Z = 17) is (2, 8, 7). Chlorine has 7 electrons in its valence shell. It can acquire a stable configuration (2, 8, 8) of the nearest inert gas (Ar) by sharing one electron with another atom.
When three chlorine atoms approach phosphorous atom, at a certain distance between the nuclei, it shares one valence electron each with the three chlorine atoms and forms three shared pairs of electrons with three chlorine atoms. The shared pair belongs to each atom equally. As only one pair is shared between the atoms, they are joined to each other by a single covalent bond.

Formation of Methane Molecule:
Electronic configuration of carbon C (Z = 6) is (2, 4). Carbon has 4 electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by sharing one electron each with four hydrogen atoms.
When four hydrogen atoms approach phosphorous atom, at a certain distance between the nuclei, it shares one valence electron each with the four hydrogen atoms and forms four shared pairs of electrons with four hydrogen atoms. The shared pair belongs to each atom equally. As only one pair is shared between the atoms, they are joined to each other by a single covalent bond.

Formation of Ethane Molecule:

Formation of Oxygen Molecule:
Consider the formation of the oxygen molecule. Electronic configuration of oxygen O (Z = 8) is (2, 6). Oxygen has 6 electrons in its valence shell. It can acquire a stable configuration (2, 8) of nearest inert gas (Ne) by sharing two electrons with another oxygen atom. The two atoms share two pairs of electrons between them completing octet of each. two shared pairs constitute a double bond.
O + O → O=O
(2,6) (2,6) (2,8) (2,8)

Formation of Nitrogen Molecule:
Consider the formation of the nitrogen molecule. Electronic configuration of nitrogen (Z = 7) is (2, 5). Nitrogen has 5 electrons in its valence shell. It can acquire a stable configuration (2, 8) of nearest inert gas (Ne) by sharing three electrons with another nitrogen atom. The two atoms share three pairs of electrons between them completing octet of each. Three shared pairs constitute a triple bond.
N + N → N≡N
(2,5) (2,5) (2,8) (2,8)

Formation of Ethene (Ethylene) Molecule:

Formation of Ethyne (Acetylene) Molecule: