Science > Chemistry > Atomic Structure > Discovery of Proton and Neutron
In last article, we have discussed, the discovery of electron and characteristics of electron. In this article, we shall discuss the discovery of proton and neutron and their characteristics.
Discovery of Proton:
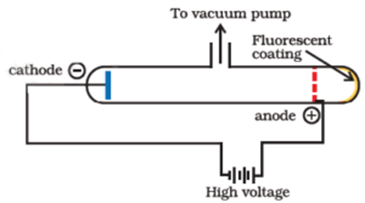
Proton was discovered by E. Goldstein in 1886. He performed the same experiment as performed by J.J. Thomson but used perforated cathode. He found that on passing an electric discharge through a gas under reduced pressure, rays containing positive particles move towards the cathode. As they appear to arise from the anode they are called anode rays or canal rays. They are found to contain positively charged particles called protons.
Origin of Positive Rays:
When an electric discharge is passed through gas at very low pressure in the discharge tube cathode rays are produced. The cathode rays consist of a stream of high-speed electrons. When these fast-moving electrons strike the atoms or molecules of the gas present in the discharge tube, they remove one or more electrons from the neutral atoms or molecules. Thus positive ions of gas are formed. These ions which move towards the perforated cathode kept midway in the tube and constitute the positive rays coming through the perforated cathode.
Characteristics of Canal Rays:
- They travel in a straight line in the opposite direction to that of cathode rays.
- Canal rays produce fluorescence when incident on zinc sulphide screen.
- Unlike cathode rays, the positively charged particles depend upon the nature of gas present in the cathode ray tube.
- These are simply the positively charged gaseous ions.
- The charge to mass ratio of the particles is found to depend on the gas from which these originate.
- Some of the positively charged particles carry a multiple of the fundamental unit of electrical charge.
- The behaviour of these particles in the magnetic or electrical field is opposite to that observed for electron or cathode rays.
Characteristics of Protons:
- Protons are positively charged.
- Protons are located in the nucleus.
- Protons have mass 0f 1.0078 a.m.u. (1.672 × 10-27 Kg.). This mass of a proton is considered as unit mass ( 1 a.m.u. ).
- Mass of one proton is almost equal to the mass of one hydrogen atom.
- The proton carries a positive charge of 1.6 × 10-19 C. This charge carried by the roton is considered to be the unit positive charge.
- Proton is denoted by 1 H 1 or 1 P 1. I.e. it has unit positive charge and unit mass.
- All atoms contain proton.
Discovery of Neutron:
In 1920 Rutherford proposed existence of the third neutral particle in an atom. Neutron was discovered by Sir James Chadwick in 1932. He observed that when Beryllium (Be) is bombarded by α -particles; particles having no charge and mass equal to that at proton were produced (ejected). He called them as neutrons.
Characteristics of Neutrons:
- Neutrons have no charge i.e. they are electrically neutral.
- They are located in the nucleus of an atom.
- Mass of neutron is of 1.008665 a.m.u (1.675 × 10-27 Kg ). For practical purpose, this mass is assumed as unit mass.
- Mass of neutron is nearly as that of the proton.
- Neutron is denoted as 0 n 1.
Other Subatomic Particles:
Mesons, positrons, neutrinos, antiprotons are other subatomic particles. Other subatomic particles discovered recently are quarks, antiquarks, pions, gluons, boson and god particle.
Discovery of X-rays:
X-Rays were discovered by Wilhelm Roentgen in 1895. He discovered that when cathode rays strike metal with a high atomic number, radiation of very short wavelength was emitted. He was unable to explain the nature of the emitted rays hence he called these rays as X-rays.
Characteristics of X-Rays:
- These rays are capable of penetrating through wood, paper, and flesh but are stopped by bones and metallic substance.
- X-Rays are electromagnetic waves.
- They are chargeless.
- X-rays produce fluorescence in many substances. The fluorescence in different substances has different characteristics.
- X-ray kill some form of animal tissues.
- X-ray affect photographic plates.
- X-rays travel by velocity of light (3 x 108 m/s) in air or vacuum.
- They are not deflected by electric or magnetic fields.
- They ionize air or gas through which they pass.
Moseley’s Contribution:
Moseley in 1913 found that each element when bombarded by high-velocity electrons, emit X-rays of different frequency. Thus the atomic number of many elements was determined accurately by the following relationship.
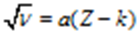
Atomic number α square root of the frequency of X-rays. He gave relation Where a and k are constant. Z is an atomic number and υ is the frequency of X-ray. Thus he stated that frequency of radiation from elements depends upon the number and arrangement of unit particles in their atoms. Hence atomic number (z) is a fundamental property of all elements.
Important Terms and Concepts:
Atomic number (Z) :
The number of protons (positive charge) present in the nucleus of an atom of a particular element is called the atomic number of that element. It is denoted by letter ‘Z’.
Neutron number (N):
The number of neutrons present in the nucleus of an atom is known as neutron number. It is denoted by ‘N’
Mass number (A):
The total number of protons and neutrons present in the nucleus of an atom of the element is called mass number. The mass number is denoted as ‘A’.
A = Z + N
Representation of Atom in Symbolic Form:
Generally, every atom X is represented as

Concept of Isotopes, Isobars, and Isotones:
Isotopes:
Different atoms of the same element having the same atomic number but having different mass numbers are known as isotopes.
Examples :

Characteristics of Isotopes:
- Different Atoms of the same element having the same atomic number but having different mass numbers are known as isotopes.
- Isotopes are the atoms of the same element.
- They have the same atomic number but different mass numbers.
- They have the same number of protons but the different number of neutrons.
- Since they have the same atomic number they show the same chemical properties.
- They occupy the same positions in the periodic table.
Isobars:
Atoms of the different elements having a different atomic number but having same mass numbers are known as isobars.
Examples :

Characteristics of Isobars:
- Atoms of the different elements having the different atomic numbers but having the same mass numbers are known as isobars.
- Isobars are the atoms of different elements.
- They have the same mass number but different atomic numbers.
- They have a different number of protons and neutrons.
- Since they have different atomic number they show different chemical properties.
- They occupy different positions in the periodic table.
Isotones:
Atoms of the different elements having the different atomic number, different mass number but having same neutron number are known as isotones.
Examples:

Characteristics of Isotones:
- Atoms of the different elements having the different atomic number, different mass number but having same neutron number are known as isotones.
- Isotones are the atoms of different elements.
- They have the different mass numbers and different atomic numbers.
- They have the same number of neutrons.
- Since they have a different atomic number they show different chemical properties.
- They occupy different positions in the periodic table.