Science > Chemistry > Atomic Structure > Discovery of Electron
In this article, we shall study earlier concepts of an atom, discovery of electron, and its characteristics.
600 B.C. Indian saint and philosopher Maharshi Kanad proposed that matter is made up of the smallest individual particles. He called these particles ‘paramanu’. Around 400 B.C. The Greek Philosopher Democritus suggested that all the matter is composed of tiny, discrete, indivisible particles. He called these particles ‘atoms’. Both the ideas are of conceptual nature and didn’t have any experimental evidence.
Assumptions of Dalton’s atomic theory:
The first concept was given by John Dalton. The postulates of his theory are:
- Every element is made up of extremely small particles called an atom.
- The atoms are indivisible and they can neither be created nor be destroyed. Atoms of the same element resemble each other in all respects but differ from the atoms of other elements.
- When chemical compounds are formed they do so by the combination of atoms of different elements in a simple proportion of whole numbers.
- Atoms of different elements may combine in more than one proportion to form different compounds.
Evidence of Electrical Nature of Matter:
When a glass is rubbed with silk or ebonite is rubbed with fur, electricity is generated. Electricity gets transferred from one point to another point through certain substances. This phenomenon indicated that the matter has electrical nature.
Michael Faraday in 1832 passed electricity through the solution and he called the phenomenon as electrolysis. He observed that charged particles migrate towards oppositely charged electrodes. During this process, they accumulate on the electrode or escape out as a gas at the electrode. On the basis of his experiments, he proposed the laws of electrolysis. These laws of electrolysis given by Michael Faraday provide a relation between matter and electricity. These laws assumed the discrete nature of electricity. These discrete particles are called electrons by Loney.
Fundamental particles of an atom:
Protons, neutrons, and electrons that make up an atom are known as the fundamental subatomic particles. Protons and neutrons are present in the nucleus of an atom, they are called intranuclear particles or nucleons. Electrons revolve around the nucleus in a circular orbit. They are called extra-nuclear particles.
Discovery of Electrons:
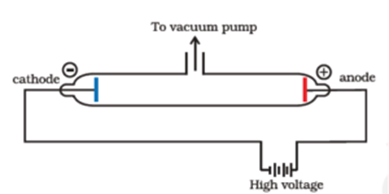
Electrons were the first of sub-atomic particles to be discovered, by J.J. Thomson in 1859. J.J. Thomson made a detailed study of the discharge of electricity through gases under very low pressure. This experiment was performed using a cathode ray tube (Crooke’s tube). It consists of a glass tube connected to two metal electrodes at two ends. There is a side tube connected to a vacuum pump to reduce pressure.
When a gas is subjected to a high potential (5000 to 10000 V) at low pressure, the glass wall of tube glows with fluorescent light. This glow is because of the bombardment of glass by rays emitted from the cathode. As the rays arise from the cathode, they are called cathode rays. These rays were found to consist of negatively charged particles with a negligible but definite mass.
Cathode rays produce heat energy when they collide with the matter. It is due to the kinetic energy possessed by the cathode rays. Cathode rays also rotate the paddle wheel in their path. They move from the negative electrode to the positive electrode. Which clearly indicate that the cathode ray consists of a negatively charged particle. Thomson called these particles as negatrons. Stoney changed this name to electrons.
Thomson also showed that the electrons are present in atoms of all elements. Thus electrons are the fundamental particles of an atom.
Charge to Mass Ratio of Electron:
J. J. Thomson measured the ratio of electrical charge (e) to mass (m) of cathode ray particles using specially designed cathode ray tube.
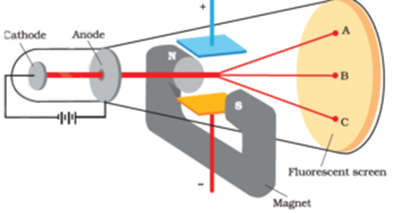
Construction of Apparatus:
The apparatus consists of a discharge tube containing gas at a very low pressure about 0.01 mm of mercury. The discharge tube has cathode C at one end and fluorescent screen S at the other. Anode A consists of a cylinder with a fine bore.
The electric field can be applied between the plates P1 and P2. Plate P1 is positive and plate P2 is negative
The magnetic field can be applied perpendicular to the electric field and perpendicular to the plane of the diagram and into the plane.
Working:
Cathode emits electrons and they are collimated by cylindrical fine bore anode. The velocity of electrons depends on the potential difference between the cathode and the anode. When no field is applied. The electrons move in a straight line and forms spot at O at the centre of the screen.
When cathode rays are passed through an electric field created by applying a potential across the plates P1 and P2 only. It is found that the cathode rays particles get deflected towards the positive plate.
When cathode rays are passed through the magnetic field created by applying a strong magnetic field only, it is found that the cathode rays particles get deflected in a circular path.
Thomson applied both the fields simultaneously and adjusted its value such that the spot remains at the centre of the screen at O. Knowing values of V, B and d and using following formula the e/m ratio can be determined.

Where E = V/x. The value of e/m is 1.758820 x 1011 C kg-16.
Thomson repeated the experiment for different materials of the cathode and found that the e/m ratio is always the same. From this, he concluded that the particles present in cathode rays (electrons) are fundamental particles of any atom of all matter.
Equation of Path of Parabolic path of Electron:
The path of an electron in the electric field is parabolic whose equation is given by

Where y = deflection of the path of the electron in the y-direction
x = Distance between the parallel plates
e = Charge on electron
E = Intensity of the electric field
m = Mass of electron
v = velocity of electron
Radius of Circular Orbit of Electron:
The radius of the circular orbit of an electron in the magnetic field is given by

Where r = radius of the circular path of an electron in magnetic fields
e = Charge on electron
B = Magnetic induction
m = Mass of electron
v = velocity of electron
Alternate Equation for e/m Ratio:

Millikan’s Oil Drop Experiment:
In the first step, the oil drops are allowed to fall between the plates in the absence of an electric field. Due to gravity, they accelerate first, but gradually velocity decreases up to certain minimum value due to air resistance. Then the drops start moving downward with a constant velocity called terminal velocity (v1). The terminal velocity of the drop is measured. As the velocity is constant, the air resistance and weight of the drop are equal in magnitude. Thus the net force acting on the drop is zero.

In the second step, an electric field is produced in the chamber. A likely looking drop is selected and kept in the middle of the field of view by adjusting the voltage. So that the drop is moving down with constant velocity called terminal velocity (v2). Thus net force acting on it is zero. Thus the electric force is balancing the downward forces.
The charge on an electron is given by

Where η = Coefficient of the viscosity of gaseous medium (air)
v1 = Terminal velocity of the oil drop when an electric field is not applied
v2 = Terminal velocity of the oil drop when an electric field is applied
E = Intensity of the electric field between plates
f = Density of oil
σ = density of the gas (air)
g = acceleration due to gravity
Millikan repeated the experiment by varying the strength of x-rays used for ionization of air no. of times. Due to which the no. of electrons attaching to the oil drop varied. Then he obtained various values for the charge and is found to be an integral multiple of 1.6 x 10-19 C.
In 1923, Millikan won the Nobel Prize in Physics in part because of this experiment.
The charge on an Electron:
Scientist R. A. Millikan in his oil-drop experiment determined the charge on the electron and he found that the charge on an electron is 1.6022 x 10-19 C.
Mass of an Electron:
Using e/m ratio and charge on the electron, the mass of an electron is found to be 9.1094 x 10-31 kg.
Characteristics of Electrons:
- Electrons are negatively charged.
- They are revolving in circular orbits around the nucleus.
- They have mass 0f 0.00055 a.m.u. (9.11 × 10 -31 Kg.). This mass of an electron is negligible compared to other particles. Hence it is taken as zero.
- Its mass is 1/1837 times that of the proton.
- Electrons carry a negative charge of 1.6 × 10 -19 C. This charge carried by the electron is considered to be a unit negative charge.
One reply on “Discovery of Electron”
Perfect