Science > Chemistry > Atomic Structure > Radioactivity
Radioactivity was discovered by French physicist Antoine Becquerel in 1896. The phenomenon of spontaneous and continuous and uncontrollable disintegration of an unstable nucleus accompanied by the emission of active radiations is called natural radioactivity. The substance which exhibits radioactivity is called a radioactive substance. e.g. Uranium, thorium, radium, etc. The radiations emitted by the radioactive substance are alpha particles, beta particles, and gamma radiations.
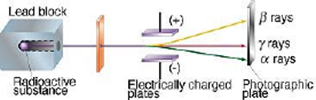
Characteristics of Natural Radioactivity:
- These characteristics are also called as Rutherford-Soddy’s radioactive disintegration theory
- Radioactivity is a purely nuclear phenomenon. The nucleus of a radioactive substance is unstable and such unstable nucleus undergoes spontaneous breakdown (disintegration). The process continues till a stable nucleus is obtained.
- As radioactivity is the nuclear phenomenon it is unaffected by chemical combination. i.e. the element will exhibit radioactivity in free as well as a combined state.
- Radioactivity is a spontaneous process. It is independent of external factors like temperature, pressure, and the state of existence of substance or catalytic action. Hence the process of radioactive disintegration is uncontrollable using these factors.
- The nucleus of radioactive element emit alpha (α), beta(β) and gamma (γ) radiations and gets converted into the nucleus of another element.
- The elements undergoing disintegration is called parent element and a new element formed is called a daughter element. Daughter element has different chemical and physical properties as compared with that of its parent element.
- During disintegration, besides emission of alpha (α), beta(β) and gamma (γ) radiation, a large amount of energy is liberated in form of γ rays. When γ rays are given out no new element is formed.
- The time taken, by a radioactive substance to disintegrate half of its initial quantity is called as half-life period. Half-life period is a characteristic property of every radio element.
- When radioactive substance emits one α -particle mass number of daughter element reduces by 4 units and atomic number reduces by 2 units. When a radioactive substance emits one β – particle, the atomic number of daughter element increases by one unit but the mass number remain unchanged.
- The rate of disintegration at any instant is directly proportional to the radioactive nuclei present at that instant.
- Thus the rate of disintegration depends on nature and the original amount of the radioactive substance. It is an exponential process and practically never gets completed.
Characteristics of α – particles:
- These are positively charged particles. So α-rays are called α – particles rather than α -rays.
- Actually, α particles are helium nuclei having 4 unit mass and 2 unit of positive charge.
- They are deflected towards a negative plate of the electric field.
- They have greater ionizing power.
- They have least penetration power.
- They can affect a photographic plate.
- They travel in straight line.
- They have a velocity which is about 1/10 th that of light.
- When radioactive substance emits one α -particle, the mass number of daughter element formed is 4 units less and the atomic number is 2 units less.
Characteristics of β-particles:
- β- Rays are negatively charged particles. So they are called β – particles rather than β – rays.
- β- particles are nothing but high-velocity electrons having unit negative charge and negligible mass.
- These rays are deflected towards + ve plate of the electric field.
- They have less ionizing power as compared with that of α- rays5. They have greater penetration power than that of α – rays
- They affect a photographic plate to the much higher extent than the α- particles.
- They do not travel in straight line.
- They have a greater velocity than that of the α- rays very close to that of light.
- When a radioactive substance emits one β -particle, the atomic number of daughter element formed is one unit higher but the mass number remains unchanged.
Characteristics of γ – rays:
- γ-rays are nonmaterial
- They are electromagnetic radiations.
- They are chargeless, hence remain undeflected due to the electric or magnetic field.
- They have very low ionizing power.
- They have high penetration power.
- They have a very little effect on a photographic plate.
- They travel in straight line.
- They have a velocity equal to that of the light.
- When radioactive substance emits γ rays there is no change in atomic number and mass number.
Distinguishing between α particles and β particles:
| α particles | β particles |
| These are positively charged particles. So α-rays are called α – particles rather than α -rays. | β – Rays are negatively charged particles. So they are called β – particles rather than β – rays. |
| Actually these particles are helium nuclei ( 2He4 ) having 4 unit mass and 2 unit of positive charge. | β – particles are nothing but high velocity electrons ( -1e0 )having unit negative charge and negligible mass. |
| They are deflected towards negative plate of electric or magnetic field. | These rays are deflected towards +ve plate of electric or magnetic plate. |
| They have greater ionising power. | They have less ionising power as compared with that of α – rays |
| They have least penetration power. | They have greater penetration power than that of α – rays |
| They can affect photographic plate. | They affect a photographic plate to much higher extent than the α – particles. |
| They travel in straight line. | They do not travel in straight line. |
| They have velocity which is about 1/10 th that of light. | They have greater velocity than that of the α – rays very close to that of light. |
| When radioactive substance emits one α -particle, mass number of daughter element reduces by 4 units and atomic number by 2 units. | When a radioactive substance emits one β -p article , atomic number of daughter element increases by one unit but mass number remain uncharged. |
Distinguishing between α particles and γ rays:
| α particles | γ rays |
| These are positively charged particles. So α-rays are called α – particles rather than α -rays. | γ-rays are non material |
| Actually these particles are helium nuclei ( 2He4 ) having 4 unit mass and 2 unit of positive charge. | They are electromagnetic radiations. |
| They are deflected towards negative plate of electric or magnetic field. | They are chargeless, hence remain undeflected due to electric or magnetic field. |
| They have greater ionising power. | They have very low ionising power. |
| They have least penetration power. | They have high penetration power. |
| They can affect photographic plate. | They have very little effect on photographic plate. |
| They travel in straight line. | They travel in straight line. |
| They have velocity which is about 1/10 th that of light. | They have velocity equal to that of the light. |
| When radioactive substance emits one α -particle, mass number of daughter element reduces by 4 units and atomic number by 2 units. | When radioactive substance emit γ rays there is no change in atomic number and mass number. |
Distinguishing between β particles and γ rays :
| β particles | γ rays |
| β – Rays are negatively charged particles. So they are called β – particles rather than β – rays. | γ-rays are non material |
| β – particles are nothing but high velocity electrons ( -1e0 )having unit negative charge and negligible mass. | They are electromagnetic radiations. |
| These rays are deflected towards +ve plate of electric or magnetic plate. | They are chargeless, hence remain undeflected due to electric or magnetic field. |
| They have less ionising power as compared with that of α – rays | They have very low ionising power. |
| They have greater penetration power than that of α – rays | They have high penetration power. |
| They affect a photographic plate to much higher extent than the α – particles. | They have very little effect on photographic plate. |
| They do not travel in straight line. | They travel in straight line. |
| They have greater velocity than that of the α – rays very close to that of light. | They have velocity equal to that of the light. |
| When a radioactive substance emits one β -particle , atomic number of daughter element increases by one unit but mass number remain uncharged. | When radioactive substance emit γ rays there is no change in atomic number and mass number. |