Science > Chemistry > Colloids > Gels
A gel is a colloidal system in which the dispersed phase is liquid and the dispersion medium is solid. e.g. when warm sol of gelatin is cooled, it sets to a semi-solid mass which is a gel. Jellies, jams, curd, butter, shoe polish, etc. are gels.
The interior (the cytoplasm) of each cell in the soft tissues of our bodies consists of a variety of inclusions (organelles) suspended in a gel-like liquid phase called the cytosol. Dissolved in the cytosol are a variety of ions and molecules varying from the small to the large; among the latter, proteins and carbohydrates make up the “solid” portion of the gel structure. The nature of gel depends on the coexistence between the solid network and the liquid medium. A gel is a soft material that can be easily cut with a knife. Removal of the liquid phase leads to the xerogel or aerogel which depends upon the drying conditions of the gel.
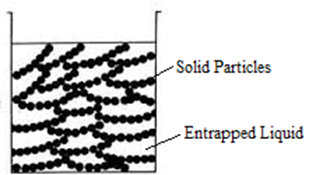
Gelation:
When a colloidal solution is coagulated, a precipitate is formed which may or may not be gelatinous. Under certain conditions, it may be possible to obtain the dispersed phase as more or less rigid mass enclosing within it all of the liquid. The product formed is called a gel and the process is known as gelation.
Characteristics of Gels:
- It is the colloidal system in which the dispersed phase is liquid and the dispersion medium is solid.
- It is an immobile semi-solid.
- It has a honeycomb-like structure.
- Many gels have a tendency to absorb liquid and swell.
- No such agent is required for its formation.
- It is classified as an elastic gel and non-elastic gel.
- They do not show the Tyndall effect, Brownian movement, and electrophoresis.
Types of gels:
Elastic Gels:
Elastic gels or reversible gels are gels which when heated carefully, form a dry mass and if this dried mass, when placed in contact with liquid, absorb liquid, swell up and regain their original form. e.g. agar-agar, gelatin, fruit jams, etc.
Characteristics of Elastic Gels:
- On heating (dehydration) they give elastic solid.
- The original gel can be obtained by addition of water or liquid to elastic solid.
- They are reversible
- They are lyophilic
- They show imbibition
- Gels, made up of organic substances are elastic.
Non Elastic Gels:
Non-elastic gels or irreversible gels are gels which when on heating loose liquid and gets converted into dry mass but cannot absorb liquid and regain their original form when they are placed in contact with the liquid. e.g. Silica gel, solid alcohol, hydroxides of Fe, Al, Cr, etc. are non-elastic gels.
Characteristics of Non Elastic Gels:
- On heating (dehydration) they give a powder.
- The original gel cannot be obtained by addition of water or liquid to powder.
- They are irreversible.
- They are lyophobic
- They do not show imbibition
- Gels, made up of inorganic substances are non-elastic.
Properties of Gels:
Swelling or Imbibition:
The tendency of a gel to take up a large quantity of water or liquid and go on increasing in volume is called swelling of gel or the smiling of gel or imbibition of gel. Only elastic gels show this property.
Syneresis:
The decrease in the volume of a gel due to the loss of liquid on standing is called syneresis or weeping of gel. Many inorganic gels on standing, undergo shrinkage which is accompanied by exudation of liquid. This process is the reverse of imbibition.
Thixotropy:
Some gels turn into a sol on shaking and reset to the gel on standing. This reversible gel-sol transformation is called thixotropy. Iron oxide and silver oxide gels exhibit this property.
Fragile Nature:
In a newly-opened container of yogurt or sour cream (gel) appears to be smooth and firm, but once some of the material has been spooned out, little puddles of liquid appear in the hollowed- portion.
It can be explained as follows. As the spoon is plunged into the gel, it pulls the nearby layers of the gel along with it, creating a shearing action that breaks it apart, releasing the liquid entrapped inside.
Hofmeister or Lyotropic Series:
The tendency of a gel to take up a large quantity of water or liquid and go on increasing in volume is called as swelling of gel or smiling of gel or imbibition of gel. Only elastic gel shows this property. Hofmeister studied the effect of a salt on swelling (imbibition) of gel. Different ions are found to have a different effect on swelling (imbibition). For example, in presence of iodide ions, the process of imbibition is so fast that the imbibition takes place even at room temperature. For other ions heating is required.
The series of cations Mg2+, Ca2+, Sr2+, Ba2+, Li+, Na+, K+, Rb+, Cs+, and of anions citrate3-, tartrate2-, SO42-, acetate–, NO3–, CIO3–, I–, CNS– (among others) is called lyotropic series or Hofmeister series. The ions of the Hofmeister series are ordered from the most (top) to least (bottom) effective in precipitating proteins out of solution.
Uses of Gels:
- Alcohol jellied with calcium acetate is used as solid fuel for military field services.
- Silica gel is most valuable adsorbing and drying (desiccating) agent which is used in industry and laboratory.
- Many articles of common use are gels. e.g. curd, fruit jams, butter, cheese, jellies, shoe polish, various foodstuffs etc.
2 replies on “Gels”
its nice note i gained knowledge thanks so much
Thanks it is so helpful for me