Science > Chemistry > Colloids > Coagulation of Colloidal Solution
The process of precipitation of colloidal particles due to aggregation of the particles is called coagulation or flocculation.
Explanation:
The presence of the same type of electric charge on colloidal particles causes repulsion and keep them in a suspended state. If by some means, the charge on the colloidal particles is removed, these particles come together, aggregated, become large enough to settle down in the form of a precipitate. Thus the charge on the colloidal particles is neutralized. In this process, the dispersion medium and dispersed phase are separated from each other.
If the precipitate formed instead of settling down, floats on the surface of the dispersion medium then the phenomenon is called as flocculation.
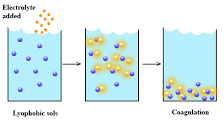
Methods of Coagulation of Lyophobic Sols:
By Mutual Coagulation or Meteral Coagulation:
If two sols of opposite charge are mixed together, their charges are mutually neutralised and both sols get coagulated.
Example:
Fe(OH)3 (+ve sol) + As2S3 (- ve sol) → Precipitate
By heating:
On the heating thermal energy of colloidal particles increases so much that it overcomes the repulsive forces between them, and particles unite to form larger particles.
The charge on colloidal particles is due to preferential adsorption. The phenomenon of adsorption is inversely proportional to temperature. Due to heating desorption of adsorbed ions from the surface of colloidal particles takes place. Similarly, the dispersion medium particles due to increase in kinetic energy disturb the surface layer of adsorbed charge.
Thus due to the above two reasons, the neutral sol particles aggregate to form a precipitate.
Example :
If an egg is boiled egg albumin gets coagulated.
Egg albumin → Precipitate.
By Electrophoresis:
Under the influence of an electric field, sol particles move to the electrode where they get discharged as a neutral particle. Neutral sol particles aggregate to form a precipitate.
Example:
Rubber from latex is coagulated by electrophoresis on cloth or metal.
By Persistent Dialysis:
On prolonged dialysis almost all the electrolyte in the colloidal solution is removed making ythe sol unstable. It leads to coagulation.
By Addition of Electrolyte:
When an excess of an electrolyte is added to a sol, oppositely charged ions of electrolyte removes the charged on sol particles. Neutral sol particles aggregate to form a precipitate. Any negatively charged sol is coagulated by the cations of electrolyte and vice-versa. The ion carrying the charge responsible for coagulation is called a flocculating ion.
Example: If BaCl2 is added to the As2S3 sol which is negative sol, Ba2+ ions causes coagulation of sol.
Flocculation Value:
The minimum concentration of an electrolyte in millimoles per litre required to cause precipitation of sol in 2 hours is called flocculation value. The smaller the flocculation value, the higher will be the coagulation power of the ion.
Hardy- Schulze Rule:
Statement: (Part – I)
The ions of electrolyte having opposite charge with respect to as that of the sol particles are responsible for the coagulation.
Example:
For positively charged sol like Fe(OH)3, the anions cause coagulation. Similarly, for negatively chargedsol like As2S3 the cations cause coagulation.
Statement: (Part – II)
Coagulating power of ions of electrolyte which cause coagulation increases with the valency (charge) of ions. More the valency of the effective ions the greater is its precipitating power.
Example :
Coagulating power of cations, coagulating any negative sol is in the order, Al3+ > Ba2+ > Na+
Coagulating power of anions, coagulating any +ve sol is in the order, PO43- > SO42- > Cl–
Coagulation of Lyophilic Sols:
Stability of lyophilic sols is due to charge and solvation of colloidal particles. Hence if these two factors are removed their coagulation can be achieved. It can be done by adding electrolyte and by adding suitable solvent like alcohol or acetone.
Protective Colloids:
Lyophilic sols are more stable than the lyophobic sols. The stability of lyophilic sols is due to the solvation i.e. a protective layer is created around the colloidal particles by the dispersion medium in which they are dispersed.
If lyophilic sols are added to lyophobic sols they form a layer around the lyophobic particles and protect them from the action of an electrolyte. Thus the lyophilic sols on the addition to lyophobic sols give extra stability to the lyophobic sol. Hence, in this case, lyophilic sols are called protective colloids.
The power of protection of lyophilic sols is measured in terms of Gold number. The number of milligrams of lyophilic colloid that will just prevent the precipitation of 10 ml of gold sol on the addition of 1 ml of 10% sodium chloride solution is a gold number. This number was introduced by Zsigmondy.
The gold numbers of some commonly used protective colloid are given below.
| Lyophilic Substance (Protective Colloid) | Gold Number |
| Gelatin | 0.005 – 0.01 |
| Haemoglobin | 0.03 – 0.07 |
| Gum Arabic | 0.15 – 0.25 |
| Egg albumin | 0.08 – 0.10 |
| Potato starch | 25 |
| Sodium oleate | 0.4 – 1.0 |
| Gum tragacanth | 2 |
| Starch | 25 – 50 |
| Sodium caseinate | 0.01 |
- Higher is the gold number, lower will be the protective power.
- Gelatin is added in the preparation of ice cream as a protective colloid for colloidal particles of ice.
- Argyrol is used as eye drops is a silver sol protected by organic material.
- Proteins in blood like haemoglobin protect the blood from coagulation due to electrolytes.
- Source of mil are human milk, cow milk, and buffalo milk. But human milk is considered the best because it is better protected than other types of milk.
Ageing:
It is a spontaneous process of destabilization in colloids by which the dispersed phase get separated from the dispersion medium on its own. (i.e. no artificial methods are used).
One reply on “Coagulation of Colloidal Solution”
keep posting like this information