Science > Chemistry > Colloids > Charge on Colloidal Particles
The colloidal particles carry an electric charge. The most important property of colloidal solution is that all suspended particles possess either positive or a negative charge. i.e. they carry the same nature of the charge. The mutual forces of repulsion between similarly charged particles prevent them from aggregating and settling under the action of gravity. This gives stability to the sol.
The dispersion medium carries the opposite charge, hence as a whole, the colloidal solution is electrically neutral.
Origin of the Charge on Colloidal Particles:
A small quantity of electrolyte is always present in the colloidal dispersion. Its presence is necessary for the stability of the sol, as complete removal of the sol causes coagulation of the sol.
Presence of Some Acidic or Basic Groups in Colloidal Solution:
Colloidal particles may acquire electric charge due to the presence of certain acidic or basic groups in colloidal solution.
For example, protein molecules give rise to the formation of colloidal solutions. Thus the particles of protein sol either have a positive charge or a negative charge depending upon the pH of the medium. A molecule of protein contains a carboxylic acid (COOH) group and also a basic amino (–NH2) group, it will form a positively charged particle in the acidic medium and a negatively charged particle in the basic or alkaline medium.
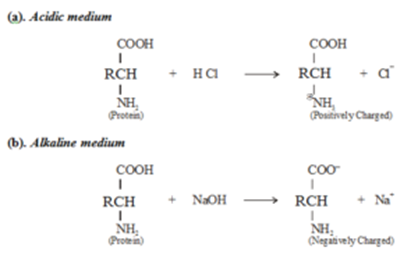
In the case of the colloidal solution of proteins, the nature of charge on colloidal particles depends on the pH of the solution. The isoelectric point of a colloid is a pH at which net charge on colloidal particles is zero. Above this pH, the particles are negatively charged and below this pH, particles are positively charged. At isoelectric point, the particles exist in the form of Zwitter ion. Hence they do not migrate under the influence of the electric field.
The isoelectric pH for some proteins is Haemoglobin (pH 4.3-5.3), Casein from human milk (pH 4.1 – 4.7), and Gelatin (pH 4.7).
Due to Self Dissociation:
The dissociation of surface molecules leads to electric charge on colloidal particles of the sol.
For example, Consider an aqueous solution of soap (sodium palmitate) which undergoes dissociation into ions.

The cations (Na+) pass into the solvent. Due to the weak attractive forces present in the long hydrocarbon chains, the anions (C15H31 COO–) have a tendency to form negatively charged aggregates of colloidal dimensions. This type of development of charge is only possible with electrolytes. This is not possible in colloidal solutions of non-electrolytes such as clay, smoke etc.
Electron Capture by Colloidal Particles:
It is believed that the colloidal solutions prepared by Bredig’s Arc Dispersion Method acquire a charge by electron capture.
Selective or Preferential Adsorption of Ions:
When two or more ions are present in the dispersion medium, then the colloidal particles adsorb preferentially positive or negative ions present in the dispersion medium.
Example: Positively charged Ferric hydroxide sol:
If FeCl3 solution is added to the Ferric hydroxide, preferentially Fe3+ ions adsorb on Fe(OH)3 molecules, that is why colloidal particles of ferric hydroxide are positively charged.
FeCl3 ⇌ Fe3+ + 3 Cl–
Fe(OH)3 + Fe3+ → Fe(OH)3 / Fe3+
Example: Positive charged Siver iodide sol:
When dilute KI is added in excess dilute AgNO3, the Ag+ ions are adsorbed on Agl, and [AgI]Ag+ is formed. Thus the colloidal particles have a positive charge.
AgNO3 + KI → AgI + KNO3
AgNO3 ⇌ Ag+ + NO3–
AgI + Ag+ → AgI / Ag+
Example: Negative charged Siver iodide sol:
When dilute AgNO3 is added in excess dilute KI, the I– ions are adsorbed on Agl, and [AgI]I– is formed. Thus colloidal particles have a negative charge.
AgNO3 + KI → AgI + KNO3
KI ⇌ K+ + I–
AgI + Ag+ → AgI / I–
Example: Negatively charged Arsenious Sulphide Sol:
It is prepared by passing H2S gas slowly through the solution of AS2O3.
AgNO3 + KI → AgI + KNO3
H2S ⇌ 2H+ + S2-
AS2S3 + S2- → AS2S3 / S2-
Frictional Electrification:
The origin of charge on the colloidal particles may be due to frictional electrification. By mutual rubbing of colloidal particles with molecules of the dispersion medium, the charge is developed on the sol. This view is not satisfactory.
Electrical Double Layer:
Colloidal particles are charged. This charge on the particle is balanced by an opposite charge in the dispersion medium. The charge in the fluid dispersion medium is in the form of free ions. There is a region around each colloidal particle where the charge on particle attracts the free ions from the dispersion medium to form an electrical cloud around it and is called the electrical double layer (Helmholtz electrical double layer).
An electric double layer consists of three regions
- Surface charge – charged ions adsorbed on the particle surface.
- Stern layer – counterions (charged opposite to the surface charge) attracted to the particle surface and closely attached to it by the electrostatic force.
- Diffuse layer – a film of the dispersion medium (solvent) adjacent to the particle. The diffuse layer contains free ions with a higher concentration of the counterions. The ions of the diffuse layer are affected by the electrostatic force of the charged particle. The boundary of this layer is called the slipping plane (shear plane).

The value of the electric potential at the slipping plane is called Zeta potential. The zeta potential is an important parameter for a colloid. Zeta potential depends on the properties of the colloid. For example, adding salt to a colloid shrinks the electrical double layer, and reduces the zeta potential. Zeta potential is given by the relation

Where, ξ = zeta potential
η = coefficient of viscosity
u = velocity of colloidal particles
D = Dielectric constant of the medium = K
Zeta potential and particle size are key indicators of the way colloids behave both in storage and in use. Zeta potential influences the effective size of the particles in the colloid.
Importance of Charge on Colloidal Particles:
- The mutual forces of repulsion between similarly charged colloidal particles prevent them from aggregating and settling under the action of gravity.
- This gives stability to the sol. In the case of lyophobic sols, charge on colloidal particles is fully responsible for its stability.
- In the case of a lyophilic sol, the stability is due to the charge on colloidal particles and solvation.
Electrophoresis or Cataphoresis:
The unidirectional migration of sol particles or dispersed phase particles or colloidal particles towards the oppositely charged electrode under the influence of the applied electric field is called electrophoresis or cataphoresis.
Cause of Electrophoresis:
All sol particle (colloidal particles) carry the same electric charge either positive or negative. If an electric potential is applied across two platinum electrodes dipping in a sol, the sol particles move towards oppositely charged electrodes.
Illustration:
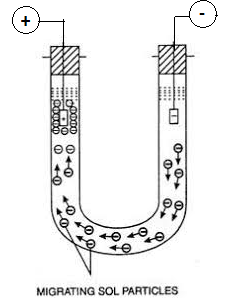
Consider a sol of As2S3 is taken in a ‘U’ shaped glass tube. The sol particles of the sol are negatively charged. Now the dispersion medium with little quantity of electrolyte is introduced over the colloidal solution. There should be a sharp boundary between the sol and the dispersion medium.

Electric potential is applied across the two platinum electrodes dipped in a sol in two limbs, it is observed that the level of sol drops at the negative electrode and rises at the positive electrode side. This shows that sol particles have migrated to the positive electrode, indicating that the particles are negatively charged.
If colloidal particles are allowed to reach the electrode, their charges are neutralised and coagulation takes place.
Applications of Electrophoresis:
- Electrophoresis is used to detect the nature of charge on colloidal particles.
- It is used in the removal of carbon particles from chimney gases.
- It is used in electro-deposition of rubber on metal, wood or cloth surfaces from latex.
- It is used to bring about coagulation of sol.
Electro-osmosis:
The migration of the dispersion medium of a colloidal solution under the influence of the electric field when the movements of colloidal particles are prevented is called as electro-osmosis.
Cause of Electro-osmosis:
Since the sol as a whole is electrically neutral, dispersion medium has an opposite electric charge as compared with that of the sol particles. If the dispersed phase has a positive charge we say that the dispersion medium has a negative charge.
Illustration:
A sol of As2S3 is filled in a glass tube. The sol particles of the sol are negatively charged. Hence the dispersion medium (water) is positively charged. The colloidal solution and pure dispersion medium in a glass tube are separated by a semipermeable membrane.

When an electric potential is applied across the platinum electrodes dipping in each arm, sol particles cannot pass through the semipermeable membrane but dispersion medium (water) move to the negative electrode through the semipermeable membrane. The level of sol drops at the +ve electrode and rises at -ve electrode. This movement of dispersion medium towards -ve electrode shows that the charge on the dispersion medium is positive.
Applications of Electro-osmosis:
- Electro-osmosis is used in dewatering of moist clay
- It is used in the drying of dye-pastes
- It is used in the removal of water from peat.
Applications of Electrical Properties of Colloids
Sewage Precipitation:
Dirty and muddy water from gutters and drainages is called sewage is in colloidal form (colloidal solution).
Sewage water containing colloidal particles of mud, rubbish etc. is collected in a tank fitted with electrodes.
On applying an electric field, colloidal particles are attracted towards oppositely charged electrodes. As their charge gets neutralised, they settle as a precipitate. The precipitated or coagulated matter called sludge is used as manure while clear water is used for irrigation.
Smoke Precipitation:
Smoke is a colloidal solution of negatively charged carbon particles in the air (aerosol)
These carbon particles may condense water vapour on them and thus cities may have a thick cover of smog (smoke + fog). This smog causes air pollution.

Cottrel’s precipitator is a widely used smoke precipitator. Smoke is passed between metal electrodes at high voltage (about 50,000 V) The charged particles are neutralized at the oppositely charged electrode and get deposited there. The gases free from carbon particles are passed to a chimney or for further purification.
3 replies on “Electrical Properties of Colloids”
Mastt
Great document
Best source of science information