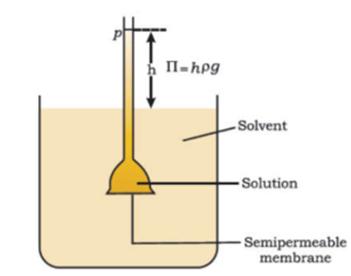
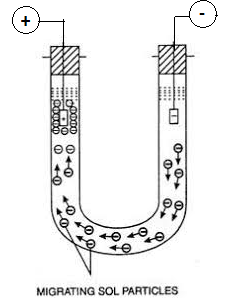
Science > Chemistry > Colloids > Charge on Colloidal Particles The colloidal particles carry an electric charge. The most important property of colloidal solution is that all suspended particles possess either positive or a negative charge. i.e. they carry the same nature of the charge. The mutual forces of repulsion between similarly charged particles prevent […]
Categories
Electrical Properties of Colloids
- Post author By Hemant More
- Post date April 20, 2020
- 3 Comments on Electrical Properties of Colloids
- Tags Brownian movement, Cataphoresis, Charge on colloidal particles, Chemistry, Colligative properties, Colloidal dispersions, Colloidal solution, Colloidal state, Colloids, Cottel's precipitator, Crystalloids, Diffuse layer, Dispersed phase, Dispersion medium, Electrical double layer, Electro-osmosis, Electron capture, Electrophoresis, Filterability, Heterogeneous character, Molecular mass, Selective preferential adsorption, Self dissociation, Sewage precipitation, Smoke precipitation, Solution, Stern layer, Surface charge, Surface tension, Suspension, True solution, Tyndall effect, Viscocity, Visibility