Science > Chemistry > Solutions and Their Colligative Properties > Osmosis and Osmotic Pressure
In this article, we shall study the phenomenon of osmosis and osmotic pressure.
Semipermeable Membrane:
A semipermeable membrane is a membrane which allows the solvent molecules, but not the solute molecules through it.
Examples: Cellulosde, cellulose nitrate, animal bladder.
Osmosis:
The spontaneous and unidirectional flow of solvent molecules through a semipermeable membrane, into a solution OR flow of solvent from a solution of low concentration to a solution of higher concentration through a semipermeable membrane, is called osmosis.
Everyday Examples of Osmosis:
- Raw mangoes when placed in a concentrated solution of common salt lose water through osmosis and ultimately shrivel into a pickle.
- Flowers revive and regain their freshness when placed in freshwater because of osmosis.
- Carrots get limed due to the loss of water to the atmosphere. But, when limped carrots are placed in water, they become firm due to the inflow of water because of osmosis.
- People consuming more salt and excessive salty food suffer from edema which is swelling and puffiness produced in the body due to retention of water in tissue cells and intracellular spaces.
- The preservation of meat and fishes against bacteria is done by salting it. The bacteria on meat or fishes lose water through osmosis and ultimately die.
- The preservation of fruits against bacteria is done by adding sugar to it. The bacteria on meat or fishes lose water through osmosis and ultimately die.
- Plants absorb water from the soil through roots due to osmosis because the root hair cells have higher osmotic pressure than that of soil water.
- Red blood cells burst when kept in water due to endosmosis.
- Blooming, opening, and closing of flowers are governed by osmosis.
- Dead bodies swell underwater due to endosmosis.
Experiment Exhibiting Phenomenon of Osmosis OR Abbe Nollet Experiment:
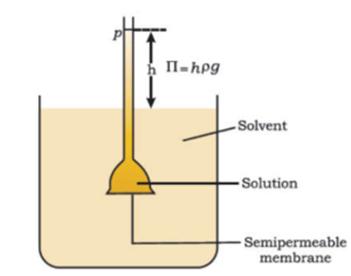
A wide-mouthed thistle funnel with a narrow long stem was taken. Then pig’s bladder (semipermeable membrane) is tied tightly around the wide mouth of the funnel with the help of a thread or rubber band. Now dilute sugar solution is carefully poured into the stem of the funnel to a certain level. The wide mouth of the funnel containing sugar solution is now kept in a beaker with the help of an Iron stand. Now three-fourths of the beaker is filled with pure water. The apparatus is left undisturbed for some time.
After a few hours we find the level of sugar solution increases from its initial level. This indicates that there is a net flow of solvent molecules into the solution through the semipermeable membrane. We have to apply excess sufficient pressure from the stem side on the solution to stop this migration of solvent molecule and this excess pressure is called osmotic pressure.
Osmotic Pressure:
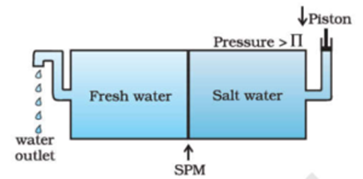
The excess of pressure on the side of a solution that stops the net flow of solvent into the solution through a semipermeable membrane is called osmotic pressure. The equilibrium is reached when hydrostatic pressure of the column is equal to that of osmotic pressure. Osmotic pressure is not created by the solution but it comes into existence when the solution is separated from the solvent by a semipermeable membrane. If the pressure applied to the solution is greater than the osmotic pressure of the solution then solvent starts passing from solution to solvent. This phenomenon is called reverse osmosis. This process is used for purification of seawater and hard water.
Types of Solutions on the Basis of Osmosis:
Isotonic Solutions:
Two or more solutions having the same osmotic pressure at a given temperature are called isotonic solutions. When such solutions are separated by semipermeable membrane no osmosis occurs between them.
For example, the osmotic pressure associated with the fluid inside the blood cell is equivalent to that of 0.9% (mass/ volume) sodium chloride solution, called normal saline solution and it is safe to inject intravenously.
Hypertonic Solution:
A solution having osmatic pressure higher than that of another solution is called a hypertonic solution.
For example, the osmotic pressure associated with the fluid inside the blood cell is less than sodium chloride solution having a concentration of more than 0.9% (mass/volume). Thus the solution of sodium chloride is hypertonic. In this case, water will flow out of the cells and cells would shrink.
Hypotonic Solution:
A solution having osmatic pressure lower than that of another solution is called a hypotonic solution.
For example, the osmotic pressure associated with the fluid inside the blood cell is more than sodium chloride solution having a concentration of less than 0.9% (mass/volume). Thus the solution of sodium chloride is hypotonic. In this case, water will flow into the cells and cells would swell.
Laws of Osmotic Pressure:
van’t Hoff’s Theory of Osmotic Pressure:
He found that the solute particles in dilute solutions possess kinetic energy and move in random directions in the solutions. Thus they have similar behaviour as that of gas molecules.
On collision against the semipermeable membrane, the solute molecules exert osmotic pressure equal to the pressure which the solute molecules would exert if it were gas molecule at the same temperature and occupying the same volume as that of solution. Thus the gas laws are equally applicable to dilute solutions.
van’t Hoff’s Boyle’s Law of Solution:
At constant temperature, the osmotic pressure (π) of a dilute solution is directly proportional to its molar concentration (C) or inversely proportional to volume (V) of the solution.
Explanation:

van’t Hoff’s Charle’s Law of Solution:
The concentration remaining constant, the osmotic pressure (π) of a dilute solution is directly proportional to absolute temperature (T) of the solution.
Explanation:

van’t Hoff’s General Solution Equation:
By van’t Hoff Boyle’s law at a constant temperature, the osmotic pressure (π) of a dilute solution is inversely proportional to volume (V) of the solution.
By van’t Hoff Charles law, The concentration remaining constant, the osmotic pressure (π) of a dilute solution is directly proportional to absolute temperature (T) of the solution.

Where k is proportionality constant called general solution constant. van’t Hoff further proved that this constant k is equal to universal gas constant R

Equations (a) and (b) represents general solution equation.
van’t Hoff’s Avogadro’s Law of Solution:
Two solutions of equal concentrations of different solutes exert same osmotic pressure at the same temperature OR equal volumes of isotonic solutions contain an equal number of solute particles at the given temperature.
Explanation:

Previous Topic: Elevation of Boiling Point and Depression in Freezing Point
For More Topics in Chemistry Click Here
One reply on “Osmosis and Osmotic Pressure”
i gained alot from this. Thank u