Science > Chemistry > Colloids > Properties of Colloids
In this article, we shall study the general, mechanical and optical properties of colloids.
General Properties of Colloids:
Heterogenous Character:
The ultramicroscopic examination indicates that colloidal dispersion is a heterogeneous system consisting of a continuous dispersion medium and discontinuous disperse phase.
Visibility:
Colloidal particles cannot be seen through naked eyes or ordinary microscope due to their very small size. The shortest wavelength in visible spectra is about 4000 Å. Hence we cannot see any object less than 200μm and colloidal particles have sizes less than 200μm.
Recently new techniques like Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM), and Scanning Transmission Electron Microscope (STEM) are used to determine the size and shape of colloidal particles.
Filterability:
The colloidal particles readily pass through ordinary filter paper. The range of particle size of colloidal substance is in between 5 × 10-9 m to 2 × 10-7 m. The pore size of ordinary filter paper is bigger i.e. of order 10-7 m. So Colloidal particles can pass through it and thus filter paper can be used to separate colloidal particles from coarse suspension.
Sols and true solutions pass through filter paper. The colloids cannot pass (diffuse) through parchment membrane but crystalloids can pass through parchment membrane. The process of separating colloids from other dissolved substance using parchment membrane as the filter is called dialysis. This process is used for purification of colloids.
When the impure sol is placed in specially created ultrafilter, with small pores, the sol particles being bigger than the pores remain behind while dispersion medium and dissolved electrolyte pass through. This process is known as ultra-purification.
Surface Tension and Viscosity:
Lyophilic sols have a higher viscosity and lower surface tension than dispersion medium and lyophobic sols have a nearly same viscosity and surface tension as the dispersion medium.
Molecular Mass:
Colloidal particles are of two types i) Multimolecular and ii) Macromolecular
- Multimolecular colloidal particles are aggregates of a number of small molecules or atoms. e.g. Sulphur sol, gold sol.
- Macromolecular particles are very big molecules or polymers. e.g. Starch, proteins.
As the colloidal particles are aggregates of a number of molecules or a large molecule themselves their molecular mass is very high.
Colligative Properties:
Colloidal particles are bigger aggregates. The colligative properties depend on the number of particles. Due to less number of particles compared to true solution colligative properties are lower. Hence the values of colligative properties like osmotic pressure., depression in freezing point and elevation in boiling points are of small order compared to the values shown by true solutions at the same concentration.
Colour:
Many sols are coloured. Sol particles are able to scatter light rays. Colour of the sol depends upon the wavelength of scattered light by the sol particles and which again depends on the size of the sol particles. The colour of colloidal solution also changes with the way the observer receives the light.
Let us consider silver sol (colloidal solution of the same substance) having different types of particles. It is found that the sols show different colours.
| Colour of silver sol | Diameter of colloidal particles |
| Violet | 15 × 10-8 m |
| Purple | 13 × 10-8 m |
| Orange-red | 9 × 10-8 m |
| Orange-yellow | 6 × 10-8 m |
Notes:
- The blue sky is due to the blue light scattered by small dust particles in the atmosphere. The atmosphere is a colloidal system consisting of dust particles suspended in air.
- The red sky is due to red light scattered by larger dust particles in the atmosphere.
- Depending on the size of the dust and water particles, different colours are seen in the cloud.
- Fine gold sol is red but as particle size increases it becomes blue or purple.
Mechanical Properties of Colloids:
Brownian Movement:
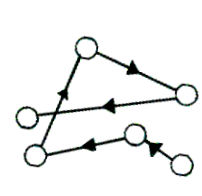
The English Botanist Robert Brown, in 1927 observed that colloidal particles exhibit continuous random motion in all directions in a straight line. He found such movement when pollen grains were suspended in water. The phenomenon of continuous zig-zag movement of colloidal particles in straight line paths in a random direction is known as a Brownian movement.
Explanation:
Colloidal particles are surrounded by a large number of dispersion medium molecules which constantly bombard the colloidal particles. On unequal bombardment, the colloidal particles get pushed in certain directions. Since colloidal particles possess like charge, they repel each other.
Factors Affecting Brownian Movement:
- Brownian movement depends on the viscosity of the dispersion medium. Brownian movement is more in less viscous solution.
- Brownian movement depends on the size of the particle. If the particles are of smaller size. The Brownian movement is more rapid.
Applications Brownian Movement:
- Due to the Brownian movement colloidal particles hardly settle down and prevent aggregation of colloidal particles. Thus colloidal solution becomes stable.
- Avogadro’s number can be calculated by Brownian movement.
Optical Properties of Colloids:
Tyndall effect:
When an intense beam of light is passed through the colloidal solution (taken in a glass vessel) placed in a dark the path of light through the colloidal solution is clearly visible due to the scattering of light by sol particles. This effect is known as Tyndall effect.
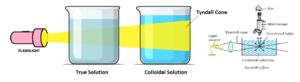

This fact was first noted in 1857 by Faraday and then studied in details by Tyndall in 1868. True solutions do not exhibit Tyndall effect.
The emitted light emerges in the form of a bright cone called Tyndall cone. Through ultramicroscope, each colloidal particle appears a bright point against the dark background, due to the scattering. Thus the colloidal particles become self-luminous. As a result, the path of the beam of light through colloidal solution becomes clearly visible. The nature of scattering depends on the size of the sol particle and the refractive indices of sol particle.
Explanation:
Colloidal particles are not large enough like suspension particles to reflect the light nor they are small enough, like true solution particles to allow the light to pass through them. Due to the intermediate size of colloidal particles, they scatter part of the absorbed light, from their surfaces in all directions. Thus the cause of Tyndall effect is a scattering of light by colloidal particles.
Conditions to be Satisfied for Viewing Tyndall Effect:
- The diameter of the dispersed particle is not much smaller than the wavelength of light used.
- There should be a large difference between the magnitudes of refractive indices of the dispersed phase and the dispersion medium.
Applications of Tyndall Effect:
- Tyndall effect is useful to distinguish colloidal solution from the true solution
- To test the purity of gases in the manufacture of H2SO4 by the contact process.
- Count the number of colloidal particles in colloidal sols using ultra-microscope.
