Science > Chemistry > Physical Chemistry > Ionic Equilibria > Ionic Theory
In this article, we shall study Arrhenius ionic theory, the concept of ionization and dissociation, Applying law of mass action to reactions involving ions.
Electrolytes on the Basis of Ionic Theory:
According to Arrhenius ionic theory, a substance (acid) base or salt, which when dissolved in water splits up spontaneously into positively and negatively charged ions and the aqueous solution has electrical conductivity is called an electrolyte e.g. Sodium chloride (NaCl), Sulphuric acid (H2SO4)
NaCl(aq) → Na+ (aq) + Cl–(aq)
H2SO4 (aq) → 2 H+ (aq) + SO42-(aq)
In modern theory, it is assumed that the solid electrolytes consist of two types of charged particles, one carrying a positive charge and other carrying a negative charge. They are held together by the electrostatic force of attraction. When such solid electrolytes are dissolved in a solvent, these forces weakened and electrolyte undergoes dissociation into ions. The process is also called ion solvation.
Non -electrolyte is a substance which in its aqueous solution or in the fused state does not conduct electricity (due to no formation of ions). Examples: sugar, urea, ethanol, starch, acetone, etc.
Types of Electrolytes on the Basis of Ionic Theory:
Strong electrolytes:
Substances which dissociate almost completely in their aqueous solutions even at moderate dilutions are called strong electrolytes. Their dissociation reaction is irreversible.
Examples:
- Strong acids like HCl, HNO3 H2SO4 etc.
- Salts like NaCl, KCl,
- Substances like H2S etc.
Characteristics of Strong Electrolytes:
- Substances which dissociate almost completely in their aqueous solutions even at moderate dilutions are called strong electrolytes.
- The degree of dissociation is high.
- The law of mass action is not applicable since dissociation is irreversible.
- Their solution has high conductivity.
- For strong electrolyte dissociation constant has a higher value.
Weak electrolytes:
Substances which dissociate to a little (limited) extent in their aqueous solutions are called weak electrolytes.
Examples:
- All weak acids like CH3COOH, HCOOH,
- all weak bases like NH4OH,
- salts like CH3 COONH4, CH3COOAg etc.
Characteristics of weak Electrolyte:
- Substances which dissociate to a little (limited) extent in their aqueous solutions are called weak electrolytes.
- The degree of dissociation is low.
- Law of mass action is applicable since dissociation is reversible.
- Their solution has low conductivity.
- For weak electrolyte dissociation constant has the lower value.
Ionisation and Dissociation on the Basis of Ionic Theory:
Ionisation:
It is the formation of the ions from molecules which are not initially in the ionic state.
Example: In HCl molecule, H and Cl atoms are covalently bonded. But when dissolved in water forms H+ and Cl– ions.
HCl(aq) ⇌ H+(aq) + Cl–(aq)
Characteristics of Ionisation:
- It is the formation of the ions from molecules which are not initially in the ionic state.
- The molecules undergoing ionisation do not contain ions of the elements forming the molecule
Dissociation:
The spontaneous splitting of a substance into positively and negatively charged ions in an aqueous solution is called dissociation.
Example: In NaCl molecule, Na and Cl atoms are bonded with an ionic bond. They exist in the ionic state even after the formation of the compound.
NaCl(aq) ⇌ Na+(aq) + Cl–(aq)
Characteristics of Dissociation:
- The spontaneous splitting of a substance into positively and negatively charged ions in an aqueous solution is called dissociation.
- The molecules undergoing dissociation contain ions of the elements forming the molecule
Degree of Dissociation (α):
The fraction of the total number of moles of an electrolyte that ionises (or dissociates) into ions in an aqueous solution at equilibrium is called as the degree of dissociation. It is denoted by ‘α’
Degree of dissociation
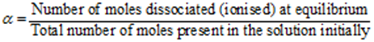
Percentage dissociation or ionisation = α × 100
Factors Affecting the Degree of Dissociation:
The degree of dissociation or ionisation depends on the following factors.
- The nature of the solute: When the ionizable parts of a molecule of a substance are held more by covalent bond than by electrovalent bond, fewer ions are furnished in the solution. e.g. H2S, HCN, CH3COOH, NH4OH, etc. When the ionizable parts of a molecule of a substance are held mainly by electrovalent bonds, more ions are furnished in the solution e.g. NaCl, KOH, etc.
- The nature of the solvent: The main function of the solvent is to weaken the electrostatic force of attraction between the ions. By Coulomb’s law, the magnitude of the force between two charged particles is inversely proportional to the dielectric constant of the medium between the charged particles. The solvent having more dielectric constant has a higher capacity of separating the ions. Water (85) > Methyl alcohol (35) > Ethyl alcohol (27) > Acetone (21). Thus water is a good solvent.
- The concentration of the solution: By Ostwald’s dilution law “The degree of ionisation of any weak electrolyte is inversely proportional to the square root of concentration and directly proportional to the square root of dilution”. Thus if the dilution increases (concentration decreases) the degree of ionisation increases.
- Temperature: Due to an increase in temperature, the kinetic energy of the molecules increases and thus attractive force between the ions in the molecule decreases, resulting in easier ionisation (dissociation). Thus if the temperature increases the degree of ionisation increases.
- It increases with dilution and also with temperature
Evidences in Favour of Arrhenius Theory:
X-ray diffraction studies have shown that electrolytes are composed of ions. For example, NaCl is present as Na+Cl–. Each Na+ ion is surrounded by six Cl– ions. In turn each Cl– ion is surrounded by six Na+ ions. A total number of Na+ ions is equal to the total number of Cl– ions. It conducts electricity in the fused state. Electrolytic solutions obey Ohm’s law. This is only possible if ions are already present in the solution. Following ionisation reaction is possible due to the existence of ions
Ag+(aq) + NO3– (aq) + Na+ (aq) + Cl–(aq) → AgCl(aq) + NaNO3(aq)
A similar reaction of AgNO3 with CCl4, CH3Cl, CH2Cl2, CHCl3 is not possible as these substances are not ionic compounds.
By Arrhenius, theory neutralization is the reaction in which H+ ion from acid and OH– ion from base react together to give practically un-dissociated water. Due to which there is a change in enthalpy of the system. This change in enthalpy is known as enthalpy of neutralization.
Abnormal behaviour of electrolytes towards colligative properties can be explained on the basis of ionic theory only. When an electrolyte is dissolved in water, the number of particles in the solution is always more than the number of molecules actually dissolved due ionisation. The van’t Hoff factor is defined as
i = Observed colligative property / Calculated colligative property
Value of i is always more than unity i.e., i = 1 + (n – 1)α
Where n is the number of ions produced from one molecule of electrolyte and α is and α is the degree of dissociation.
Colour of electrolytic solutions is due to the presence of ions. Ionic theory successfully explains the concept of common ion effect, solubility product, hydrolysis, electrolysis, the conductivity of electrolytic solutions etc.
Expressions for Dissociation Constants:
Expression for the Dissociation Constant of an Acid (Ka):
Let ‘HA’ be a weak acid. In an aqueous solution, it dissociates to a limited extent and equilibrium exists as,
HA + H2O ⇌ H3O+(aq) + A–(aq)
By applying the law of mass action to above equilibrium we have
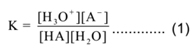
Now water is present in large excess as a solvent, its concentration can be assumed to be constant. Thus [H2O] = constant. Now the molar concentration of hydronium ion and hydrogen ion is the same,
hence, [H3O+] = [H+]
Hence the equation (1) can be written as
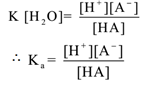
Where “Ka” is the dissociation constant of the acid.
The ratio of the product of the molar concentration of ions formed to the molar concentration of unionised acid molecule at equilibrium is called the dissociation constant of an acid. The value Ka is expressed in terms of moles/dm3. The greater is Ka value the stronger is the acid.
Expression for the Dissociation Constant of a Base (Kb):
Let ‘BOH’ be a weak base. In an aqueous solution, it dissociates to a limited extent and equilibrium exists as,
BOH + H2O ⇌ B+(aq) + OH–(aq)
By applying the law of mass action to above equilibrium we have

Now water is present in large excess as a solvent, its concentration can be assumed to be constant. Thus [H2O] = constant.
Hence the equation (1) can be written as

Where “Kb” is the dissociation constant of the base.
The ratio of the product of the molar concentration of ions formed to the molar concentration of unionised base molecules at equilibrium is called the dissociation constant of a base. The value of Kb is expressed in terms of moles/dm3. The greater is Kb value the stronger is the base.
Strength of Acids and Bases:
Strong Acids:
The acid which dissociates almost completely and produces a large number of H+ ions in aqueous solution is called a strong acid.
Examples: HCl , HNO3 , H2SO4 , HCIO4, etc.
Characteristics of Strong Acids:
- The concentration of H+ ions is more
- pH of the solution in water is nearly zero.
- They dissociate completely in water, hence α = 1 or nearly equal to 1
- They have a high value of dissociation constant ka
Weak Acids :
The acid which dissociates to a small (limited) extent and produces a small number of H+ ions in an aqueous solution is called a weak acid.
Examples: HCN, HCOOH, CH3COOH, H2CO3, etc.
Characteristics of Weak Acids:
- The concentration of H+ ions is less.
- pH of the solution in water is nearly seven.
- They do not dissociate completely in water, hence α is nearly equal to 0 and ( 1 – α) is nearly equal to 1.
- They have a low value of dissociation constant ka
Strong Bases:
A base which dissociates almost completely and produces a large number of OH- ions in an aqueous solution is called a strong base.
Examples: NaOH, KOH, etc.
Characteristics of Strong Bases:
- The concentration of OH– ions is more.
- pH of a solution in water is nearly 14.
- They dissociate completely in water, hence α = 1 or nearly equal to 1
- They have a high value of dissociation constant kb
Weak Bases:
A base which dissociates to a small (limited) extent and produces a small number of OH– ions in an aqueous solution is called a weak base.
Examples: NH4OH, Ca(OH)2
Characteristics of Weak Bases:
- The concentration of OH– ions is less.
- pH of the solution in water is nearly seven.
- They do not dissociate completely in water, hence α is nearly equal to 0 and ( 1 – α) is nearly equal to 1.
- They have a low value of dissociation constant kb
Monobasic or Monoprotic Acid:
Acids like HCl, CH3COOH are called Monobasic or Monoprotic acids. One molecule of these acids produces one H+ ion hence they are called Monobasic or Monoprotic acid.
For Monobasic acid, Equivalent weight = Molecular weight
Normality = Molarity
Dibasic or Diprotic Acid:
Acids like H2SO4 is called Dibasic or diprotic acids. One molecule of these acids produces two H+ ions hence they are called dibasic or diprotic acid.
For dibasic acid, Equivalent weight = Molecular weight / 2
Monoacidic Base:
Bases like NaOH, KOH are called Monacidic bases. One molecule of these bases produces one OH- ion hence they are called Monacidic bases.
For Monoacidic base, Equivalent weight = Molecular weight
Normality = Molarity
Diacidic Base:
An acid like Ca(OH)2 is called diacidic base. One molecule of these bases produces two OH- ions. Hence they are called a diacidic base.
For diacidic base, Equivalent weight = Molecular weight / 2
Other Important Formulae Used in Numericals of Ionic Theory:


Expressing Strength of a Solution:
Decimolar solution = 0.1 M solution
Semimolar solution = 0.5 M solution
Decinormal solution = 0.1 N soution
Seminormal solution = 0.5 N solution
M/5 solution = 1/5 M solution = 0.2 M solution
N/2 solution = 1/2 N solution = 0.5 N solution
Preferential Discharge Theory:
If an electrolytic solution contains more than two ions and electrolysis is done, it is observed that all the ions are not discharged at the electrode simultaneously but certain ions are liberated at electrodes in preference to other. This phenomenon can be explained on the basis of preferential discharge theory.
It states that if more than one type of ions are attracted towards a particular electrode, then the one discharged is the ion which requires the least energy. The potential at which the ion is discharged or deposited on the appropriate electrode is called discharge or deposition potential. Discharge potential is different for different ions.
Example:
In the case of NaCl in water, there are two equilibria Thus there are four ions involved.
NaCl(aq) → Na+ (aq) + Cl–(aq)
H2O → H+ (aq) + OH–(aq)
Now discharge potential of H+ is lower than that of Na+. Hence at cathode H+ ions will get discharged preferably. Similarly, the discharge potential of Cl- ion is lower than OH- ions. Hence at the anode, Cl– ions will get discharged preferably. Thus Na+ and OH– ions remain in solution and when the solution is evaporated crystals of sodium hydroxide (NaOH) are obtained.
The decreasing order of discharge potential for cations is K+ > Na+ > Ca+2 > Mg+2 > Zn+2 > H+ > Cu+2 > Hg +2 > Ag+ . The decreasing order of discharge potential for anions is SO4-2 > NO3– > OH– > Cl– > Br– > I–Note: When Hg is used as cathode, Na+ ions have lower discharge potential than H+ ions.
4 replies on “Ionic Theory”
I really appreciate
I am very grateful
That’s wonderful
Bravo!!!