Science > Chemistry > Chemical Equilibrium > Law of Mass Action
In this article, we shall study the law of mass action and its application to chemical equlibrium.
Rate of Chemical Reaction:
The change in the concentration of the reactants (or products) per unit time is called the rate of reaction Mathematically it can be expressed as, The rate of Reaction = Change in Concentration of Products or Reactants/time in which change is taking place Hence the rate of chemical reaction is the change in concentration of the reactants in unit time. It’s S.I. unit is mol dm-3 s-1.
Active Mass:
Active mass is the molar concentration per unit volume of that substance. It is denoted by enclosing the symbol or formula of that substance in the square bracket. For solutions, it is expressed in “ moles dm-3”. For gases, it is expressed in “ mol dm-3” or pressure in the atmosphere (atm) or bar or pascal (Pa).
Factors Affecting the Rate of Chemical Reaction:
Concentration:
If the concentration of reactants increases then the rate of reaction increases.
Pressure:
Change in pressure plays an important role in gaseous reactions. There can be three types of gaseous reactions:
Reaction with the increase in volume:
The reduction in pressure for a gaseous reaction accompanied by an increase in volume increases the rate of reaction.
e.g. PCI5(g) ⇌ PCl3(g) + Cl2(g)
Reaction with the decrease in volume:
The increase in pressure for a gaseous reaction accompanied by a decrease in volume increases the rate of reaction.
e.g. N2(g) + 3 H2(g) ⇌ 2NH3(g)
Reaction with no change in volume:
The gaseous reaction of equilibrium, involving no change in volume is independent of pressure.
e.g. H2(g) + Cl2(g) ⇌ 2HCl(g)
Temperature:
In general rate of reaction increases with increase in temperature.
Catalyst:
In a chemical reaction, catalyst increases the rate of reaction.
Light:
The reactions which take place in the presence of light are called photochemical reactions. Such reactions are influenced by light.
Size of particles:
If solid reactants are used in powdered form instead of granular form, the rate of reaction increases due to an increase in the surface area available for reaction
Law of Mass Action:
In 1864, Norwegian chemists Cato Guldberg and Peter Wage put forward the law of mass action
Law of mass action states that “The rate of a chemical reaction is directly proportional to the product of active masses of reactants, at given temperature at that instant”.
Where active mass is molar concentration per unit volume of that substance. It is denoted by enclosing the symbol or the formula of that substance in the square bracket and expressed in mol dm-3.
Explanation of Law of Mass Action:
Consider a hypothetical reaction
A + B → Products.
According to the law of mass action the rate of reaction
R ∝ [A] [B].
For any general reaction
aA + bB → Products.
According to the law of mass action the rate of reaction
R ∝ [A]a [B]b
Equilibrium mixture:
The mixture of reactants and products formed at the chemical equilibrium state is called equilibrium mixture.
Equilibrium Concentrations:
The concentrations of the reactants and products in a chemical equilibrium state for a reversible reaction are called equilibrium concentrations.
The Expression for Equilibrium Constant in Terms of Concentrations:
Consider a hypothetical reversible reaction
A + B ⇌ C + D
According to the law of mass action the rate of the forward reaction
Rf ∝ [A] [B]
∴ Rf = Kf [A] [B]
Where Kf = rate constant for the forward reaction
According to the law of mass action the rate of the backward reaction
Rb ∝ [C] [D]
∴ Rb = Kb [C] [D]
Where Kb = rate constant for the backward reaction
Now, for a chemical equilibrium,
Rf = Rb
Kf [A] [B] = Kb [C] [D]
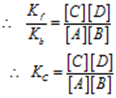
Where KC is known as equilibrium constant and the equation is called “mass law equation”.
Consider a general reversible reaction
aA + bB + cC + … ⇌ lL + mM + nN + …., Then,
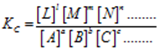
The equilibrium constant ‘ KC‘ is defined as the ratio of the product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficients of the substance in the balanced chemical equation.
Concentration is expressed in terms of mol dm-3 or mol L-1 or M. The equilibrium constant is denoted by Kc.
More is the numerical value of ‘K’ then more is the concentration of products in comparison with reactants & vis-a-vis.
Concentration Quotient of a Chemical Reaction:
At a given temperature, the ratio of the product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficients of the substance in the balanced chemical equation is called concentration ratio or concentration quotient. It is denoted by QC.
At equilibrium, the concentration ratio is equal to the equilibrium constant Kc.
Its significance is that it helps in the prediction of the direction in which the net reaction is proceeding at given concentrations or partial pressures of reactants and products.
The generalization is
- If QC > KC: The reaction is taking place in a backward direction i.e. in the direction of reactants.
- If QC < KC: The reaction is taking place in a forward direction i.e. in the direction of products.
- If QC = KC: The reaction is in an equilibrium state and hence no net reaction is taking place.
Law of Chemical Equilibrium:
At a given temperature, the ratio of the product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficients of the substance in the balanced chemical equation has a constant value.
The Expression for Equilibrium Constant in Terms of Partial Pressure:
Partial Pressure:
It is the pressure exerted by a gas in a mixture of gases if it alone occupies the entire volume of the mixture of gases. The partial pressure of a gaseous component is proportional to the mole fraction. The partial pressure of a gas is calculated using the following formula
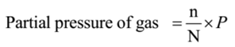
Where, n = Number of moles of gaseous component
N = Total moles of a gaseous system
P = Total pressure of the gaseous system
The Expression for Equilibrium Constant:
In the gaseous system, the concentrations in concentration quotients are replaced by partial pressure, because for given temperature the partial pressure of the gas is directly proportional to its concentration.
Consider a hypothetical reversible reaction
aA(g) + bB(g) ⇌ cC(g) + dD(g)
Then the equilibrium constant in terms of partial pressures is given by
