Science > Chemistry > Solutions and Their Colligative Properties > Solutions and Their Types
In this article, we shall study the concept of solutions and their types based on phases, composition, etc.
The Terminology of Solutions:
- Solution: A solution is a homogeneous mixture of two or more than two or more components. A solution has a single phase. The constituent particles of solution can not be separated by filtration, settling or centrifugal action.
- Solvent: The component of a solution which dissolves the other component in itself is called a solvent. It is the larger component of the solution. For example, a solution of sugar in water is solid in the liquid. Here, sugar is the solute and water is the solvent.
- Solute: The component of the solution which dissolves in the solvent is called the solute. The solute is the smaller component of the solution. For example, a solution of sugar in water is solid in the liquid. Here, sugar is the solute and water is the solvent.
- Homogeneous Solution: If the composition and properties are uniform throughout the mixture, the solution is called a homogeneous solution.
- If the solution consists of two chemical components, the solution is called a binary solution. If it contains three or four chemical components it is called a ternary or quaternary solution.
- Soluble Substance: A substance that dissolves in a solvent is said to be soluble in that solvent. A soluble substance is able to dissolve in a solvent because attractive forces between the solvent and solute particles are strong enough to overcome the attractive forces holding the solute particles together.
- Insoluble Substance: A substance that does not dissolve in a solvent is called insoluble in that solvent.
- Miscible Liquids: Two liquids that are soluble in each other are said to be miscible such as water and vinegar, coffee and cream
- Immiscible Liquids: Liquids that are not soluble in each other are immiscible such as vegetable oil and vinegar, petrol and water.
- The Process of Dissolving: Solvent particles surround solute particles to form a solution in a process called solvation. If the solvent is water the process is known as hydration. During this process, there is a change in energy observed by an increase or decrease in temperature.
- Like dissolve Like: Polar dissolves polar & nonpolar dissolves nonpolar.
- Aqueous Solution: Solutions having water as a solvent are called aqueous solutions.
The concept of Solubility:
When a substance (solute) gets dissolved in a liquid (solvent) to form a solution it means solute-solvent interaction is greater than solute-solute. The solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent. It depends upon the nature of the solute and solvent as well as temperature and pressure.
Let us consider the effect of these factors in a solution of a solid or a gas in a liquid. When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is known as dissolution. Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallization. A stage is reached when the two processes occur at the same rate. Under such conditions, the number of solute particles going into the solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached. At this stage, the concentration of solute in solution will remain constant under the given conditions, i.e., temperature and pressure. A similar process is followed when gases are dissolved in liquid solvents.
Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution. An unsaturated solution is one in which more solute can be dissolved at the same temperature. The maximum amount of solute that dissolves completely in a given amount of solvent at constant temperature is called the solubility of the solute.
Factors that affect solubility:
- The solubility of a solid in liquid increases with the increase in temperature. Whereas, the solubility of a gas in the liquid decreases with the increase in temperature.
- A particular volume of solvent can dissolve some maximum amount of solute. Thus the rate of dissolving decreases with the increase in the amount of solute.
- Stirring or agitating solution increases the rate of dissolving. Agitation increases the rate by bringing fresh solvent into contact with more solute
- Dissolving occurs at the surface of a solid, by increasing the surface area we can increase the rate of dissolving.
TYpes of Solutions:
On the Basis of Size of Solute Particles:
Depending upon the size of solute particles, the solutions are broadly classified into three types.
- Corse Solution: When the size of constituent particles is relatively bigger, then the solution is called a coarse solution e.g. a mixture of salt and sugar.
- Colloidal Solution: A colloidal solution is formed when the size of the particles dispersed in the solvent is in the range of 10-7 cm to 10-4 cm. The colloidal particles carry a charge of the same nature, which is important for the stabilization of the solution e.g. ferric hydroxide sol
- True Solution: A true solution is defined as a homogeneous mixture of two or more substances, the composition of which is not fixed and may be varied within a certain limit. The size of the particles dispersed in the solvent is less than 10-4 cm. e.g. Solution of common salt in water.
Characteristics of True Solutions
- A solution is a homogeneous mixture
- The size of solute particles in the solutions is extremely small. It is less than 1 nm in diameter.
- The particles of a solution cannot be seen even with a microscope.
- The particles of a solution pass through the filter paper. Thus filtration cannot separate the solution.
- It is very stable. The particles of solute present in a solution do not separate out on keeping.
- A true solution does not scatter light (because its particles are very small).
On the Basis of Phases of Solute and Solvent:
Depending upon the phases of solute and solvent the solutions are broadly classified into three types.
Gaseous Solutions:
- Solute phase: solid, solvent phase: gas, Also known as solid in gas solutions e.g. iodine in air
- Solute phase: liquid, solvent phase: gas, Also known as a liquid in gas solutions e.g. Chloroform in nitrogen
- Solute phase: gas, solvent phase: gas, also known as gas in gas solutions e.g. Mixture of non-reacting gases (O2+ N2)
Liquid Solutions:
- Solute phase: solid, solvent phase: liquid, Also known as solid in liquid solutions e.g. sugar in water
- Solute phase: liquid, solvent phase: liquid, Also known as a liquid in liquid solutions e.g. ethanol in water
- Solute phase: gas, solvent phase: liquid, Also known as gas in liquid solutions e.g. carbon dioxide in water
Solid Solutions:
- Solute phase: solid, solvent phase: solid, Also known as solid in solid solutions e.g. Alloys like brass, bronze
- Solute phase: liquid, solvent phase: solid, Also known as a liquid in solid solutions e.g. Amalgam of mercury with metal
- Solute phase: gas, solvent phase: solid, Also known as gas in solid solutions e.g. pumice stone, H2 gas in palladium
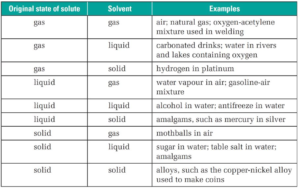
Sometimes solutions of liquid in gas and solid in a gas are not considered as solutions because the mixture may not be homogeneous.
On the Basis of Concentrations:
- Saturated solution: A saturated solution is defined as the solution that contains a just amount of dissolved solute necessary for establishing equilibrium between dissolved solute and undissolved solids.
- Unsaturated solution: An unsaturated solution is defined as a solution in which more solute can be dissolved at the same temperature.
- Supersaturated solution: A supersaturated solution is defined as a solution in which excess solute is dissolved than required for the formation of a saturated solution
Next Topic: Solid and Liquid Solutions

3 replies on “Solutions and Their Types”
Nice study material
Thanks for this
It is very easy to learn and easy to study and thanks for give these point
so easy and informative