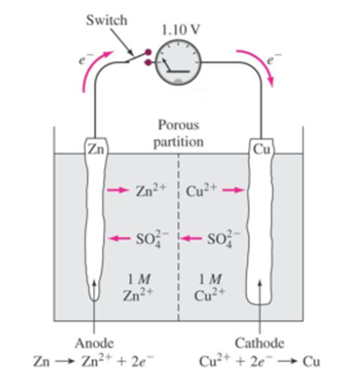
Science > Chemistry > Electrochemistry > Electrolysis and its Applications A process in which electrical energy is converted into chemical energy by using a suitable device known as an electrolytic cell is called electrolysis. Electrolysis of Fused NaCl: Construction of Electronic Cell or Electrolytic Cell: The electrolytic cell consists of a vessel made up of […]
Categories
Electrolysis and its Applications
- Post author By Hemant More
- Post date December 23, 2019
- No Comments on Electrolysis and its Applications
- Tags Anion, Anode, Cathode, Cation, Concept of overvoltage, Construction of electrolytic cell, Coulomb, Daniel cell, Determination of chemical equivalent, Determination of equivalent mass, Dry Cell, Electrochemical cell, Electrode, Electrolysis of aqueous NaCl, Electrolysis of fused NaCl, Electrolysis of NaCl solution, Electrolyte, Electrolytic cell, Electrometallurgy, Electroplating, Electrorefining, Galvanic cell, Half cell reaction, Lead accumulator, Leclanche cell, Net cell reaction, Net reaction, Non-electrolyte, Overall reaction, Oxidation, Redox reaction, Reduction, Voltaic cell, watt