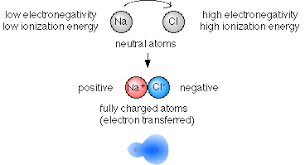
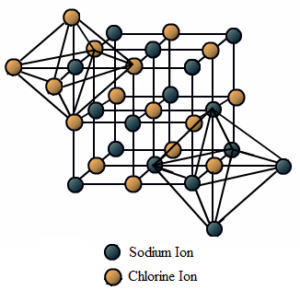
Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Properties of Ionic Compounds In this article, we shall study the properties of ionic compounds. Physical State: Due to strong electrostatic force present between the oppositely charged ions, they are held closer and fixed at specified positions in the crystal lattice. Hence ionic […]
Categories
Properties of Ionic Compounds
- Post author By Hemant More
- Post date March 31, 2020
- No Comments on Properties of Ionic Compounds
- Tags Atomic number, Bond, Born-Haber cycle, Chemistry, Coordinate bond, Covalent bond, Dash formula, dash structure, Dot formula, Dot structure, Duplet, Electrical conductivity, Electron affinity, Electron gain enthalpy, Electronegative atom, Electronic configuration, Electropositive atom, Electrovalent bond, Expanded octet, Geometry of molecule, Hydration energy, Incomplete octet, Inert electron pair effect, Ionic bond, Ionic reactions, Ionization energy, Lattice energy, Lattice enthalpy, Lewis structure, Nature of chemical bond, Octet, Octet theory, Stereoisomerism, Valence electrons, Valency orbit, Variable electrovalency