Science > Chemistry > Solid State > Classification of Crystalline Solids In this article, we have to study classification of crystalline solids and characteristics of each type. Broadly crystalline solids are classified into 4 types. a) molecular solids, b) ionic solids, c) metallic solids, and d) covalent solids Molecular Solids: Molecular solids are crystalline solids […]
Author: Hemant More
Science > Chemistry > Solid State > Introduction to Solid State There are three states of matter, solid, liquid and gaseous. Liquids and gases are called fluids because of their ability to flow. The fluidity in both of these states is due to the fact that the molecules are free to move about. The free mobility […]
Science > Chemistry > Chemical Thermodynamics and Energetics > Gibb’s Energy and Spontaneity The Concept of Gibb’s Free Energy (G): By the second law of thermodynamics, STotal = ΔSSystm + ΔSSurroundings Thus to decide spontaneity of the process we have to determine the change in entropy of the system and the change in entropy of the surroundings. […]
Science > Chemistry > Chemical Thermodynamics and Energetics > Concept of Entropy of a System In this article, we shall study the concept of entropy and its relation with the spontaneity. Spontaneous Process: The spontaneous process is defined as a process that takes place on its own without external influence. A spontaneous process does not […]
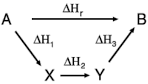
Science > Chemistry > Chemical Thermodynamics and Energetics > Hess’s Law and its Applications In this article, we shall study Hess’s law and its applications in thermochemistry. Statement : It states that the change in enthalpy accompanying a chemical reaction is independent of the pathway between initial and final states of the chemical reaction. Explanation: […]
Bond Enthalpy
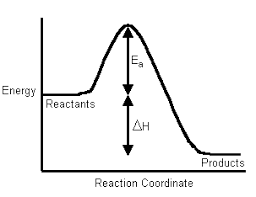
Science > Chemistry > Chemical Thermodynamics and Energetics > Bond Enthalpy In this article, we shall study the concept of bond enthalpy. During the formation of the bond, energy is evolved or released. Thus the bond formation reaction is exothermic. Due to the release of energy molecule formed is at a low energy level and […]

Science > Chemistry > Chemical Thermodynamics and Energetics > Heat of Reaction Of Different Processes In this article, we shall study change in enthalpy for different chemical processes. Enthalpy of Formation (ΔfH° or ΔformationH°): The change in enthalpy of a chemical reaction at a given temperature and pressure, when one mole of the substance is formed […]
Heat of Reaction
Science > Chemistry > Chemical Thermodynamics and Energetics > Heat of Reaction The branch of chemistry which deals with the quantitative study of thermal or heat changes in various chemical reactions is known as thermochemistry. In this article, We shall discuss very important concept of chemistry i.e. heat of reaction. Thermochemical Equation: An equation which […]

Science > Physics > Interference of Light > Numerical Problems on Fringe Width In this article, we shall study numerical problems based on Young’s experiment and biprism experiment to find the fringe width of the interference pattern and to find the wavelength of light used. Example – 12: In a biprism experiment, the slit is […]
Numerical Problems on Fringe Width

Science > Physics > Interference of Light > Numerical Problems on Fringe Width In this article, we shall study numerical problems based on Young’s experiment and biprism experiment to find the fringe width of the interference pattern and to find the wavelength of light used. Example – 01: In Young’s experiment, the distance between the […]